Team:Calgary/Project/HumanPractices/Killswitch/KillGenes
From 2012.igem.org


Hello! iGEM Calgary's wiki functions best with Javascript enabled, especially for mobile devices. We recommend that you enable Javascript on your device for the best wiki-viewing experience. Thanks!
Kill Genes: An active approach

Background
In our active kill switch, two novel nuclease enzymes work in tandem to cause fine degradation of the bacterial genome.
Nuclease genes in the 2011 parts registry were insufficient for design parameters in our project for two reasons: one, these enzymes did not provide sufficiently fine genome degradation; and two, the operating temperatures of these enzymes are unsuitable for tailings pond conditions. To this end, we submitted S7 micrococcal nuclease and CviAII into the parts registry.
S7 micrococcal nuclease
S7 nuclease is native to Staphylococcus aureus. S. aureus uses this enzyme to destroy extracellular DNA when it infects humans. S7 has both endo and exonuclease activity. This enzyme has a preference for -AT rich regions as opposed to -GC rich regions. However, this enzyme digests the DNA into <200 bp fragments. Ideally this enzyme will be present both intracellularly and extracellularly. We synthesized this enzyme from IDT. However this came with a mutation which altered a lysine residue to an isoleucine thereby making the enzyme dysfunctional.
CviAII restriction enzyme
CviAII is a restriction endonuclease that was sourced from the Chlorella virus PBCV-1 (Zhang et al., 1992). Our team selected this enzyme for three reasons. Firstly, this enzyme recognizes small, four-base pair restriction sites as opposed to other restriction enzymes such as the six-base cutter BamHI from the 2007 Berkely team (BBa_I716462). Henceforth, the CviAII restriction site is 16 times more prevalent in the E. coli genome and causes finer degradation of genetic material. Secondly, CviAII is able to cut Dam and Dcm methylated sites in the E. coli genome, and this decreased selectivity increases prevalence of cut sites.Finally, the temperature optimum for CviAII functionality is 23 degrees Celsius (Zhang et al., 1992). This optimum is closer to temperature conditions in the tailings ponds, and thus, CviAII will exhibit better enzyme activity as opposed to other enzymes in the registry with higher operating temperatures.
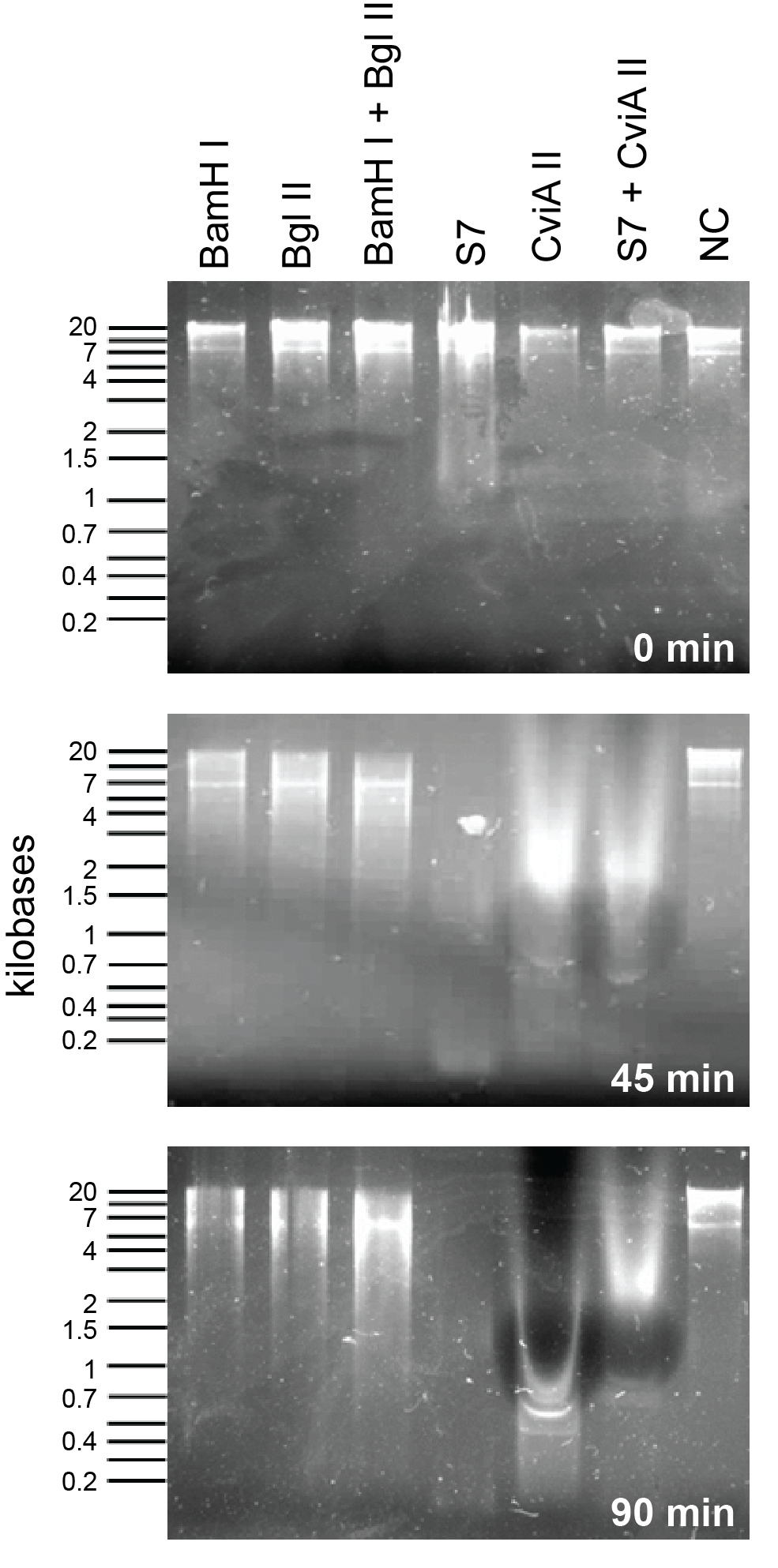
Nuclease assay: our nucleases versus those in the 2011 registry (BglII and BamHI):
In order to compare the S7 and CviAII to other nucleases in the 2011 registry, we used combinations of commercial enzymes from New England Biolabs against E. coli genomic preps. Please view the nuclease assay protocol. As shown in Figure one, S7 activity is extremely rapid and shows degradation at the zero time point. Following 45 minutes of incubation time, S7 and CviAII have chewed up the E. coli genome into small fragments whereas BamHI and BglII treated fragments are significantly larger. After 90 minutes, S7 and CviAII have sheared the genome into pieces <200 bp in size whereas there is no difference in the lanes with BglII and BamHI are similar to the 45 minutes time point.
 "
"