Team:Calgary/Project/HumanPractices/Killswitch/KillGenes
From 2012.igem.org


Hello! iGEM Calgary's wiki functions best with Javascript enabled, especially for mobile devices. We recommend that you enable Javascript on your device for the best wiki-viewing experience. Thanks!
Kill Genes: An active approach

Background
When designing our actual kill genes, we needed to consider again the challenges of our environment. Many of the restriction enzymes found in the registry such as BamHI and BglII are active only at temperatures around 37° C. Although our bioreactor may be at this temperature, the surrounding environment would be much cooler. Since the kill genes should be active in the surrounding environment, we needed to pick enzymes that would be active at lower temperatures. In addition to that we also wanted enzymes that would cut very frequently in the E. coli genome to limit the chance of horizontal gene transfer. Finally, as we chose to use an inducible system, which can easily be mutated, we wanted to introduce some redundancy by using two different kill genes.
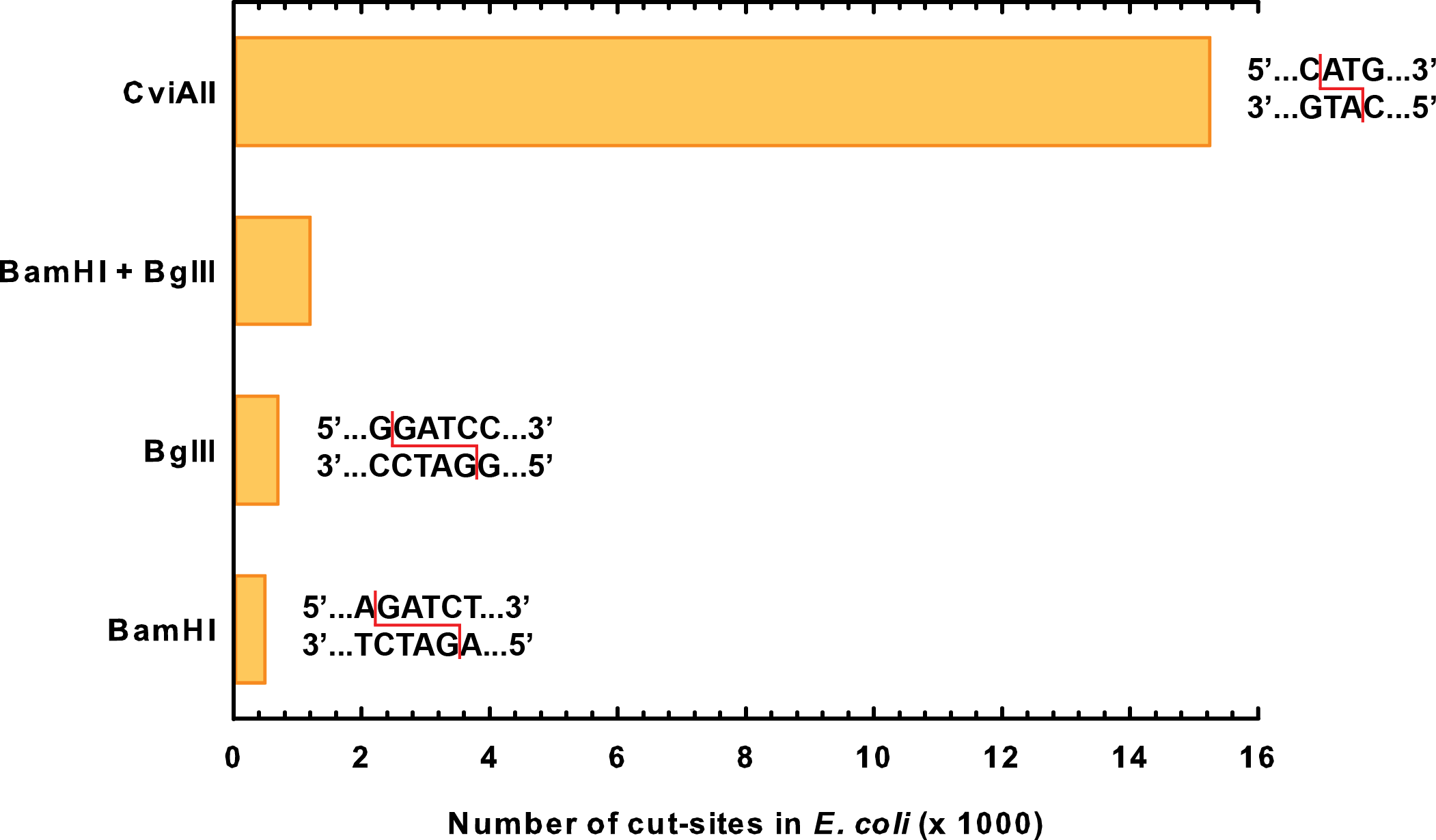
We ended up choosing genes for two novel kill enzymes: S7 micrococcal nuclease (BBa_K902019) and CviAII (BBa_K902022). Both of these enzymes are active at much lower temperatures than restriction enzymes. Through sequence analysis of the E. coli genome, we also determined that they cut extremely frequently in the genome, much more so than BglII or BamHI even combined (Figure 1).
Kill Gene: S7 micrococcal nuclease
S7 nuclease is native to Staphylococcus aureus. S. aureus uses this enzyme to destroy extracellular DNA when it infects humans to evade the immune system. S7 has both endo and exonuclease activity, and has a preference for -AT rich regions as opposed to -GC rich regions. Due to the non-specificity and high activity of this enzyme, it digests the DNA into <200 bp fragments. Ideally this enzyme will be present both intracellularly and extracellularly. The intracellular fraction would degrade the E. coli genome and the extracellular fraction would degrade any free floating DNA thereby reducing the chances of horizontal gene transfer. We synthesized this enzyme from IDT, however it came with a mutation which altered a lysine residue to an isoleucine making the enzyme dysfunctional. In order to overcome this issue, constructs with S7 were subjected to site-directed mutagenesis to restore the activity of the enzyme (Dingwall et al., 1981).
Kill Gene: CviAII Restriction Enzyme
CviAII is a restriction endonuclease that was sourced from the Chlorella virus PBCV-1 (Zhang et al., 1992). Our team selected this enzyme for three reasons. Firstly, this enzyme recognizes small, four-base pair restriction sites as opposed to other restriction enzymes such as the six-base cutter BamHI from the 2007 Berkely team (BBa_I716462). Because of this, the CviAII restriction site is 16 times more prevalent in the E. coli genome and causes more thorough degradation of genetic material. Secondly, CviAII is able to cut Dam and Dcm methylated sites in the E. coli genome, and this decreased selectivity increases prevalence of cut sites. Finally, the temperature optimum for the enzyme is 23°C (Zhang et al., 1992). This optimum is closer to temperature conditions in the tailings ponds, and thus, CviAII will exhibit better enzyme activity as opposed to other enzymes in the registry with higher optimal temperatures.
Nuclease Assay
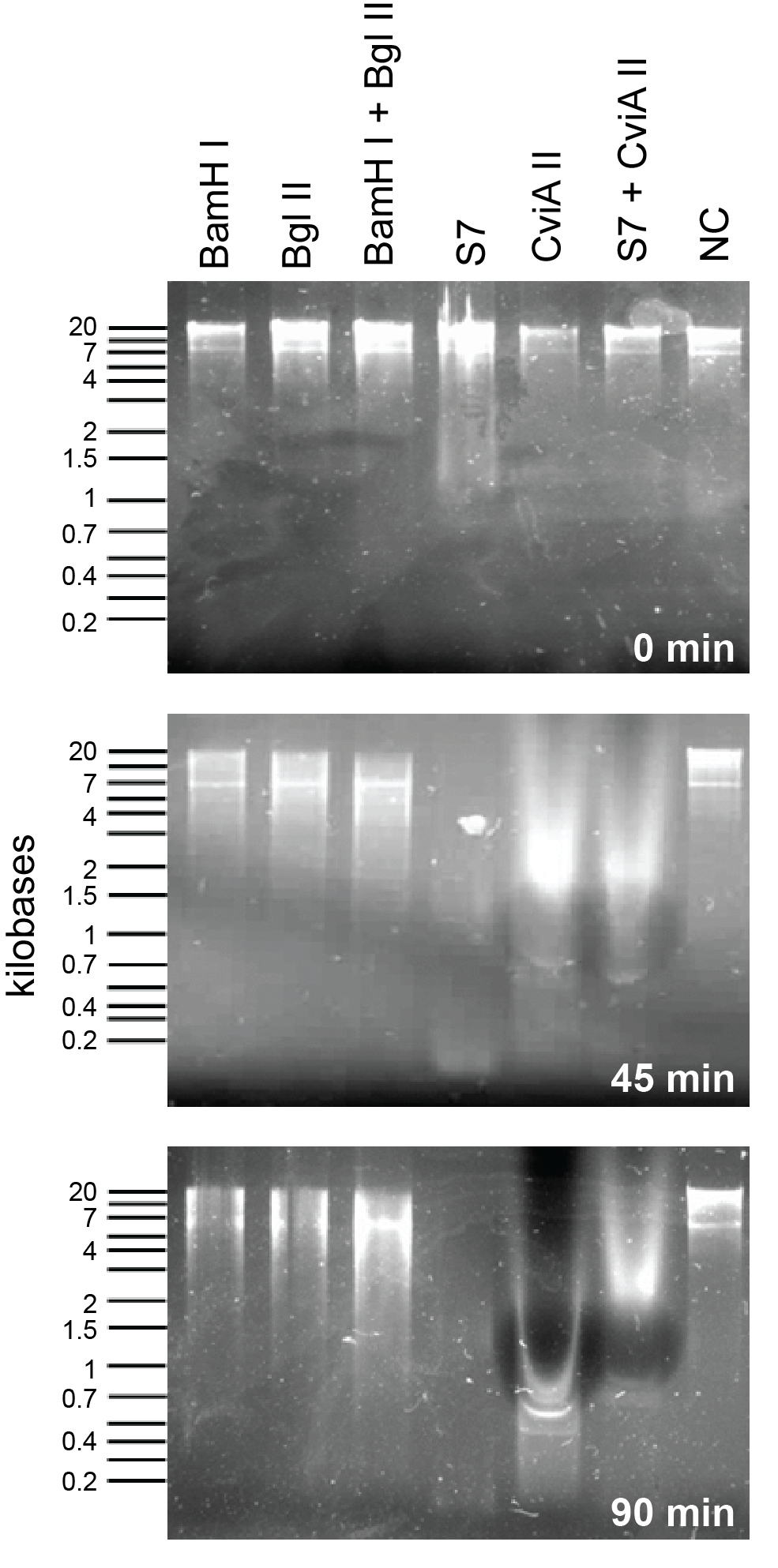
In order to compare the S7 and CviAII to other nucleases in the 2011 registry (BglII and BamHI), we used combinations of commercial enzymes from New England Biolabs to digest E. coli genomic preparations. Please view the nuclease assay protocol for more details on how this was done. Activity of S7 nuclease is extremely rapid and shows degradation at the zero time point (Figure 2). Following 45 minutes of incubation time S7 and CviAII have digested the E. coli genome into small fragments, whereas BamHI and BglII treated fragments are significantly larger. After 90 minutes, S7 and CviAII have sheared the genome into very small fragments (less than 200 bp in size) while there are no difference in the lanes with BglII and BamHI which are similar to the 45 minutes time point (Figure 2).
 "
"