Team:Calgary/Project/DataPage
From 2012.igem.org


Hello! iGEM Calgary's wiki functions best with Javascript enabled, especially for mobile devices. We recommend that you enable Javascript on your device for the best wiki-viewing experience. Thanks!
Detect and Destroy: Data Page
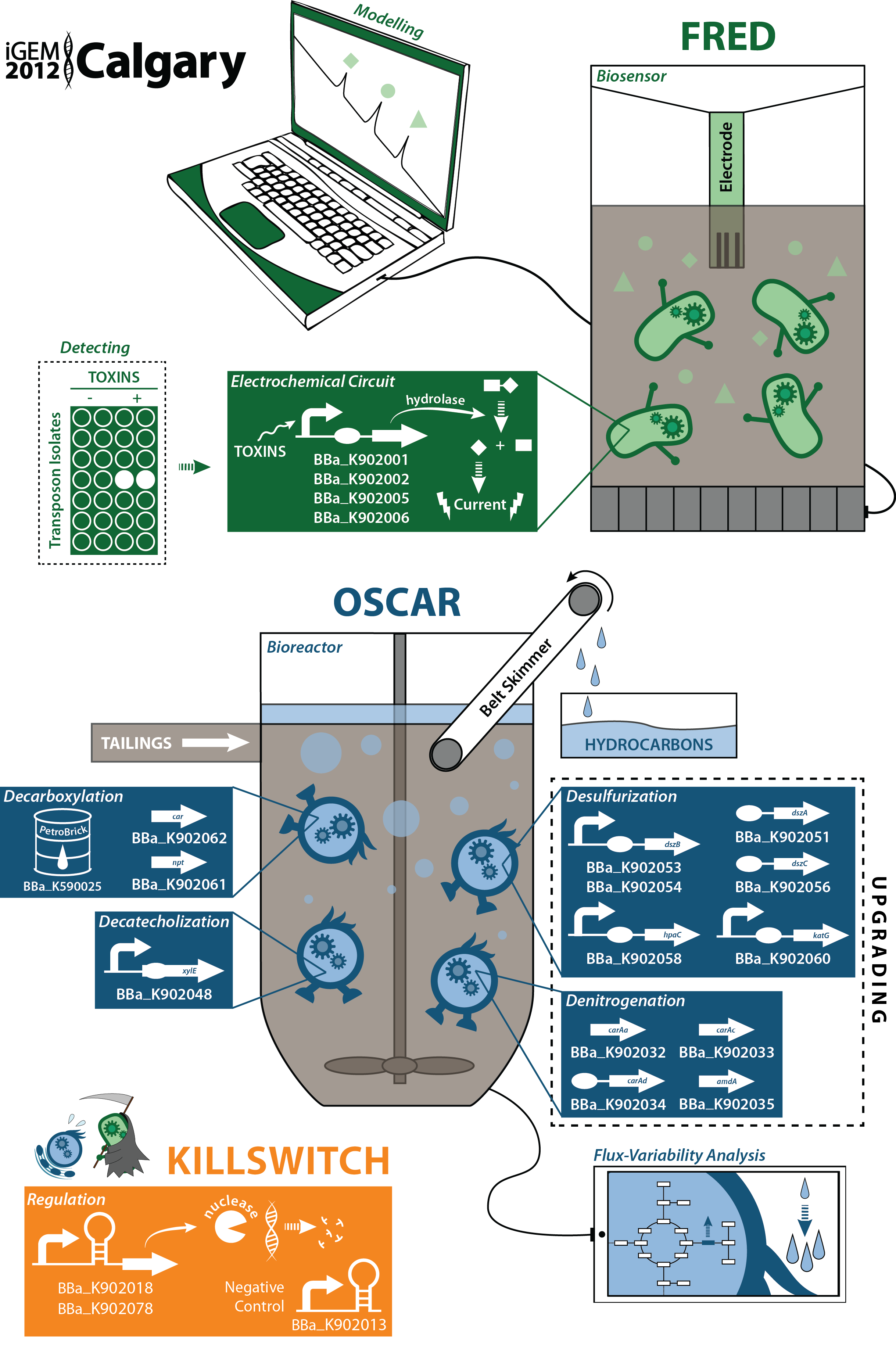
Characterization of new parts submitted to the Registry
(BBa_K902000) and (BBa_K902004): two novel hydrolase enzymes were submitted to the registry for the hydrolysis of two different sugar-conjugated electroactive compounds: PNPG and PDPG. Used in conjunction with the existing lacZ part in the registry (BBa_I732005) which hydrolyzes CPRG, this allows for the electrochemical detection of three compounds with a single electrode. A uidA inducible generator (BBa_K902002) was submitted and characterized electrochemically. This data can be found on our Electroreporting page.
(BBa_K902008),(BBa_K902023) and (BBa_K902074): three novel riboswitches sensitive to magnesium, molybdenum cofactor, and manganese were submitted along with two associated promoters (BBa_K902009 and BBa_K902073) in addition to a rhamnose inducible, glucose repressible (Prha) promoter (BBa_K902065).
The magnesium riboswitch (BBa_K902008) was tested with GFP and a constitutive promoter using this construct, (BBa_K902021), with its promoter and GFP using this construct (BBa_K902017) and with its promoter and the S7 kill gene using this construct (BBa_K902018). This data can be found on our killswitch Regulation page.
The Prha promoter was characterized via fluorescence output using a GFP composite part (BBa_K902066). This promoter was additionally characterized with our S7 kill gene as a composite part (BBa_K902084). This data can be found on our killswitch Regulation page.
Genes for denitrogenation and desulfurization were biobricked and submitted. The amdA, amidase gene (BBa_K902041) was biobricked and shown to be able to remove primary amides from a variety of compounds. A novel oxidoreductase part (BBa_K902058) was also submitted and its functionality characterized for use in the desulfurization project. This data can be found on our upgrading Desulfurization page.
Further characterization of parts already present within the registry
The IPTG inducible lacI regulated promoter (BBa_R0010) was tested electrochemically to demonstrate its leakiness when not used in conjunction with strong expression of regulatory elements. This data can be found on our Electroreporting page.
A β-galactosidase (LacZ) inducible generator construct existing in the registry (BBa_I732901) was found to possess a frameshift mutation, affecting its functionality. This part was replaced with a new circuit (BBa_K902090), which was characterized for functionality both qualitatively as well as electrochemically. This data can be found on our Electroreporting page.
(BBa_K590025), the PetroBrick, submitted by the Washington team in 2011, was characterized for a novel function: the conversion of naphthenic acids and 2-hydroxymuconate- a catechol break-down product from from the xylE gene (BBa_J33204) into hydrocarbons and potential value added products. This data can be found on both the Decarboxylation page and the Decatecholization page. We feel that these new and meaningful applications of this part present a distinct improvement on its usefulness for other teams.
The output of (BBa_K590025) was also optimized through a program we developed in MATLAB for the optimization of metabolic pathways in synthetic biology metabolic networks. The program allows you to build an artificial synthetic biology network in E. coli and predicts substrates that should be fed to the organism to increase production of the compound. This was characterized and validated in the wetlab with the Petrobrick. This data can be found on our Flux Analysis page.
An existing xylE gene in the registry (BBa_J33204) was constructed with a constitutive promoter instead of the glucose-repressible part available within the registry. This allows for increased output in media containing glucose, making it more suitable for a variety of applications such as our own. We validated the functionality of this part which can be found on our Decatecholization page. In addition, we documented a novel application for this part, by using it in conjunction with Washington's PetroBrick (BBa_K590025) to degrade catechol into a further break-down product.
An E. coli catalase gene from the registry (BBa_K137068) was also tested in conjunction with a lacI inducible promoter as a new composite part (BBa_K902060) . This part was characterized in TOP10 E. coli for its ability to allow cells to survive in higher concentrations of hydrogen peroxide. This data can be found on our Desulfurization page.
Additional Work and Characterization
Developed and tested both hardware and software for a biosensor using an electrochemical sensor. The software is available on our wiki as are the results fom the hardware. These are on our Device Prototype page
Characterized one of our constitutively expressed transposon clones to test the lacZ gene electrochemically. In addition, one of our two 'toxin-sensing' transposon hits was characterized electrochemically, demonstrating its ability to respond to and report on NAs at levels detectable by our electrochemical reporting system. This data can be found on our Electroreporting and Synergy pages respectively.
Performed an actual test of our biosensor using tailings pond water, showing that we can detect toxins found in tailings. In addition, performed an actual "field test" of our prototype to demonstrate its feasibility and ease of use outside a laboratory setting. This data can be found on our Synergy page.
Submitted novel parts involved in decarboxylation and validated the functionality of an additional enzyme (oleT), capable of converting fatty acids into alkenes by itself. This was done in its host organism. This data can be found in our Decarboxylation section, however this gene has not yet been submitted due to problems cloning it.
Designed and prototyped a physical bioreactor for which we obtained both qualitative and quantitative data for its functionality. This is outlined on our Bioreactor page. In addition, we performed an actual validation assay of our bioreactor, showing that we can use it to grow hydrocarbon producing cells and use our belt skimming device to harvest the hydrocarbons. This data can be found on our Synergy page.
Characterized the biodegradation of carbazole and various sulfur-containing compounds resembling naphthenic acids in the organisms from which we got our genes. This data can be found on our (Desulfurization) and (Denitrogenation)
Performed initial assays on a glycine knockout strain of E. coli, characterizing its survival in differing concentrations of glycine, its ability to work in conjunction with one of our inducible killswitch constructs (BBa_K902018) and finally its ability to work with the Petrobrick, actually substantially increasing our out put of hydrocarbons when grown with glycine as compared to a DH5alpha strain. This data can be found on our Synergy page.
Resubmitted (BBa_K26009) an inconsistent registry composite part that we had to construct from basic parts, resubmitting as the sequence-verified (BBa_K902016)
 "
"