Team:Calgary/Project/Synergy
From 2012.igem.org


Hello! iGEM Calgary's wiki functions best with Javascript enabled, especially for mobile devices. We recommend that you enable Javascript on your device for the best wiki-viewing experience. Thanks!
Synergy: Putting it all Together

Incorporating human practices in the design of our system
In the earlier stages of our project, we realized that in order to give our project the best chance of being implemented, we needed to do it in a way that was in line with both industry’s wants and needs. To ensure that we did this, we established a dialogue with several experts in order to get their opinions on how we should approach our project. This led to an informed design of our system, in which we emphasized the need for both physical and genetic containment devices.
Have we accomplished our goal?
Nearing the end of our project however, we wanted to see if we had accomplished what we set out to do. So we decided to go back to the experts, this time taking the progress we’ve made on our project with us. We got a variety of different perspectives from suggestions on the...... The results of all of these can be found on our Interviews page. One major concern was scale-up. One expert wanted to know how feasible this system would actually be. We have some FRED components, we have OSCAR components, and we have some killswitch components, but how functional are some of these parts, and how do they work together. So our next major goal was to establish synergy: try to put some of these pieces together in order to assess how far we’d actually gotten.
Putting FRED together
Now that we’ve been able to show that we can indeed sense three compounds electrochemically and simultaneously using our hydrolase system, our next goal was to actually try to sense toxins. Despite the fact that we have encountered significant difficulty in trying to sequence our transposon clones, given hat
Can we sense toxins in tailings ponds?
Putting together our killswitches
Our flux-based analysis allowed us to realize the potential for glycine to be used not only as a way to increase the yield of OSCAR, but also as an auxotrophic killswitch. This allowed our model to be used not only to inform our wetlab, but also our human practices. We wanted to see how this auxotrophic marker system could work with one of our inducible killswitch constructs. So we transformed our rhamnose inducible killswitch construct containing S7 (BBa_K902084) into our glycine knockout strain and attempted to characterize cell death over a variety of conditions.
Putting our Killswitch into OSCAR
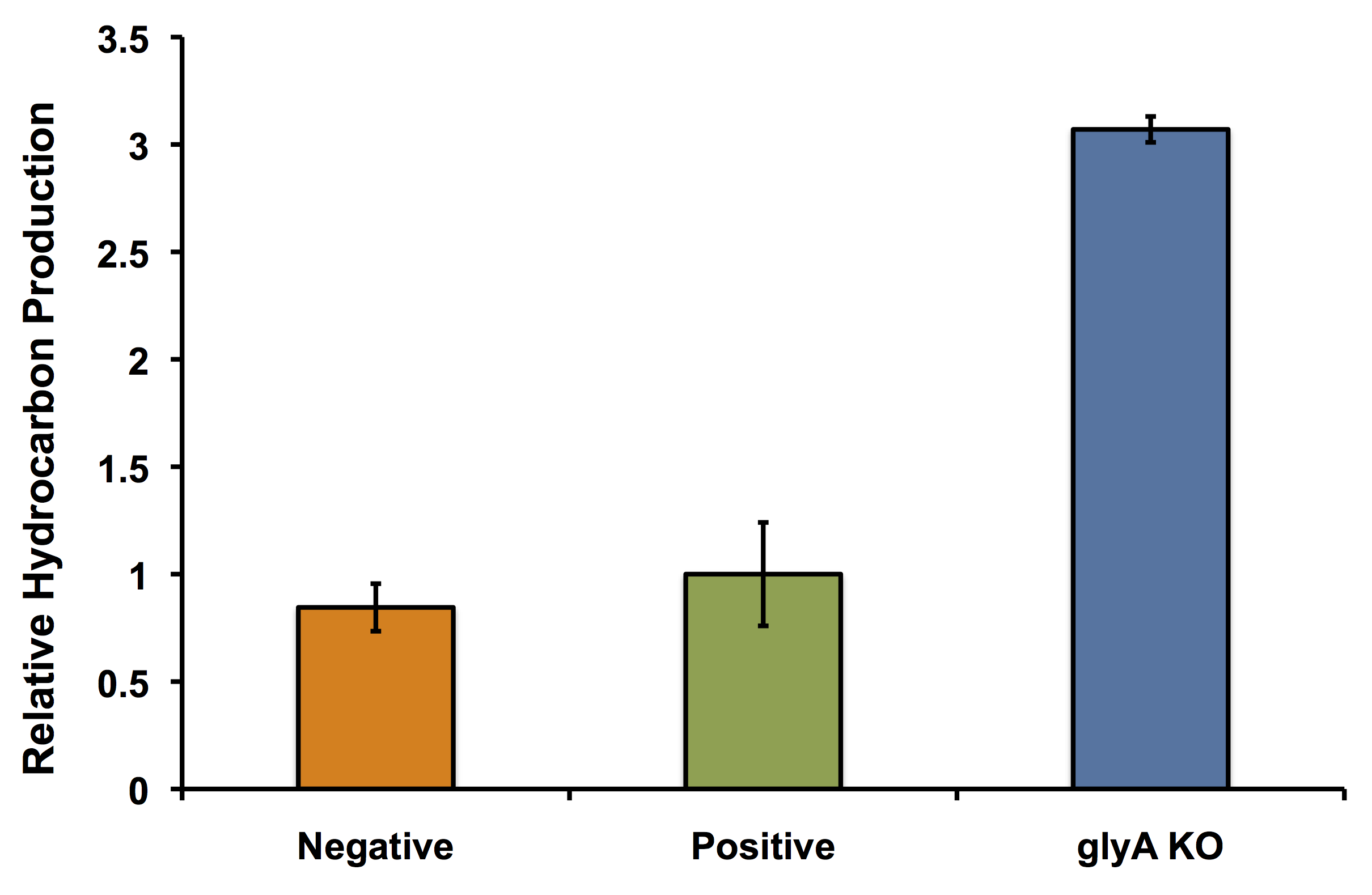
The next thing we wanted to validate was that our glycine knockout strain would in fact work as we wanted it to in OSCAR. Namely, we wanted to know if putting the PetroBrick into our glycine knockout strain and growing it in the presence of glycine would still give us the same increased hydrocarbon production that we saw when validating our model. We transformed the PetroBrick into the knockout strain and repeated the PetroBrick validation assay protocol. Our results are shown below:
Taking FRED out to the field!
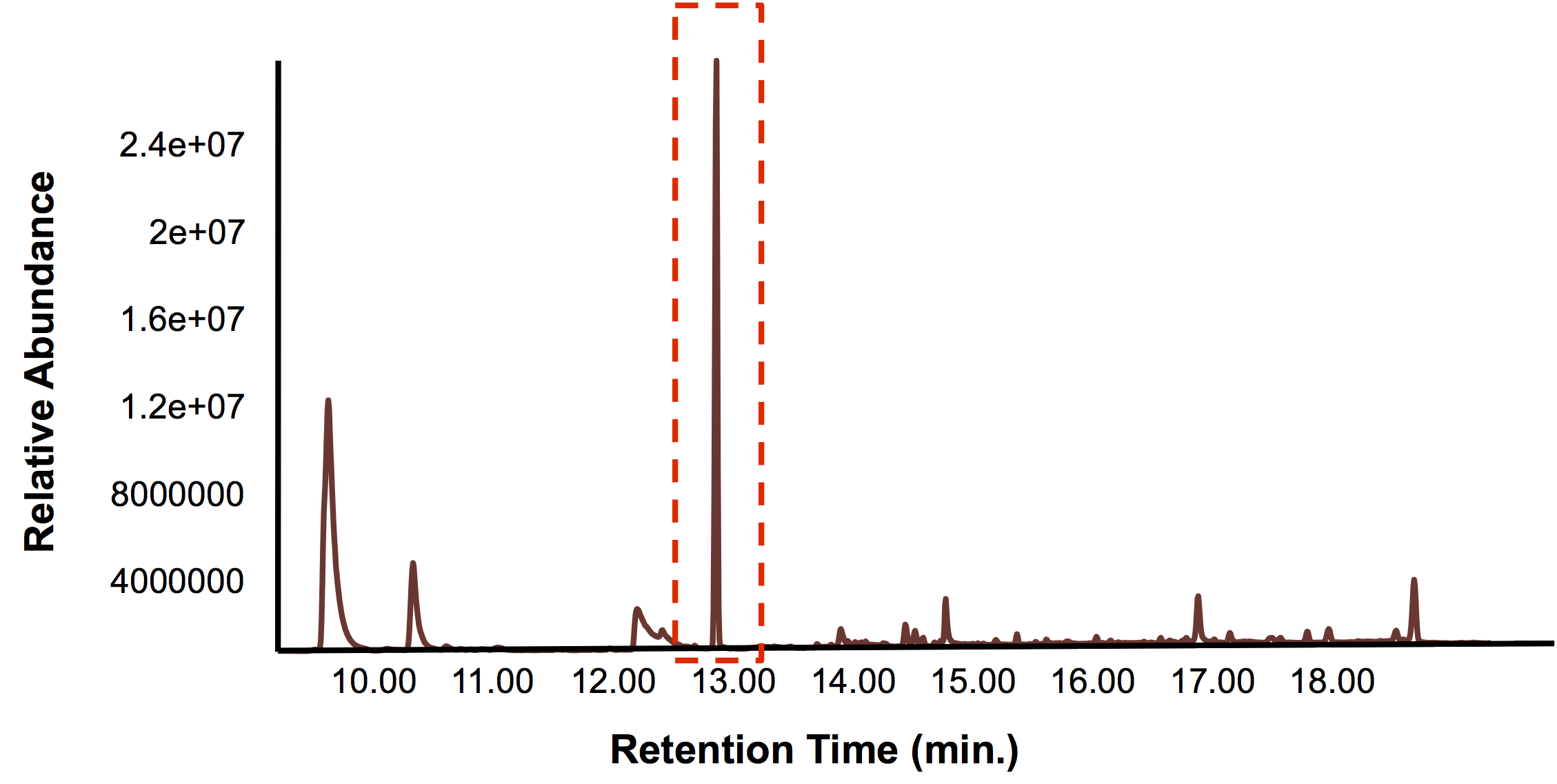
Once we knew that we had a promoter/reporter system that could actually detect toxins found in tailings ponds within the laboratory, the next challenge was to detect tailings pond toxins with our FRED prototype on site. Unfortunately, there are very strict regulations surrounding tailings ponds, and the publication of information pertaining to their contents. As such, obtaining permissions for a tailing pond field test was not possible within the time frame of our project. Because we did want to to perform a kind of field test with FRED, we investigated whether it would be permissable or advisable to try FRED outside of the lab. We performed a literature search to look for any regulations that might exist. Nothing pertaining to our province could be found, so we looked to Ontario and the United States. The concise guide to U.S. federal guidelines, rules and regulations for synthetic biology outlined the rules pertaining to field tests and indicated that in cases where organisms are going to be released into the environment, the EPA (environmental protection agency) requires a TSCA (Toxic Substances Control Act) Experimental Release Application (TERA) to be completed 60 days before the trial begins and the APHIS (Animal and Plant Health Inspection Service) requires a permit or notification. Although we specifically designed FRED to not release the microbes but rather to contain them, the prototype is too much in its infancy to remove it from the lab and be absolutely assured that it won’t be released. However, we could test tailings water with our biosensor prototype in the lab. Here is the data for this test.
Putting OSCAR in action!
Once we had tested FRED and shown that we could use him to detect toxins in tailings samples we wanted to put OSCAR into action in his home the bioreactor. By the end of the summer, we had designed and built a lab scale prototype of our bioreactor system. However, to better understand the needs of the oil sands industry we approached Kelly Roberge, an oil sands consultant specializing in tailings ponds. Through speaking with Mr. Roberge, we were able to better understand the concerns that the oil sands industry has with the use and building synthetic biology systems to solve the challenges they face. In particular, Mr Roberge had questions that surrounded the feasibility of scaling up our bioreactor to an industrial scale. As it turns out there are a number of considerations that should be made when moving from the lab scale to industrial scale. Particularly, because these transitions can be an imperfect when moving from the lab scale to industrial scale (1000L+ tanks). To start with, we would need to consider the amount of naphthenic acids needed to provide steady throughput in our system and just how much hydrocarbon can be produced in a full cycle of our system. He recommended that we use computer modelling to explore these challenges. This could allow us to determine the possible hydrocarbon output of our lab scale experiments once they are up and running. Additionally, we would need to take into consideration the composition of tailings pond solution, especially the sludge and bitumen content. The sludge could be physically harmful to our bioreactor and reduce its overall efficiency as well. A possible way to tackle this challenge would be to use current mature fine tailings drying techniques used to help speed the reuse of water in the tailings ponds. As tailings fines settle the resulting tailings water component would be left behind. This would be an ideal input into our system for potential remediation and production of hydrocarbons as it would contain a large proportion of the compounds thought to be most toxic in the tailings. By using this matured tailings as the input to our system it could help increase the efficiency of our bioreactor and provide for a smoother scale up from the lab bench to an industrial bioreactor.
Glycine Auxotrophy Still Allows For Hydrocarbon Production
In fact its better! The glycine auxotroph will be used as a second layer of regulation with our kill switch in the event that our bacterium is capable of escaping the bioreactor. However in order to ensure that the glycine knockout we are using does not compromise the production of hydrocarbons and we can continue to see the high yield of hydrocarbons as predicted with our flux balance modelling, we performed an experiment to look at the relative amount of hydrocarbon production as in the flux balance analysis model. As seen in the figure below, using the glyA knockout greatly increased the output of hydrocarbons much higher than in the wild type E. coli strain. This was extremely exciting showing that our system could not only be safe, with a second layer of control for safety, and an increase in output.
 "
"