Team:Calgary/Project/FRED/Reporting
From 2012.igem.org
| Line 57: | Line 57: | ||
<h2>What Next?</h2> | <h2>What Next?</h2> | ||
| + | |||
| + | <p>As we have shown in figure 5C, we can accurately and extremely quickly detect the level of expression of a promoter. The ability to do this in a matter of minutes allows multitudes of promoters to be characterized in an extremely short span of time. Being able to quantitatively detect extremely low expression also gives credit to the idea that electrochemical reporters are a usable standard for detecting levels of promoter expression. Having one reporter constitutively expressed as a population counter, a second as a negative control, and the third as the promoter reporter would create a robust procedure with built in controls for measuring promoter expression.</p> | ||
<p>With our electrochemical system functioning properly we can now hook up our reporter genes to promoters found in the <a href="https://2012.igem.org/Team:Calgary/Project/FRED/Detecting">transposon library</a> for a final detection system. We have also created a <a href="https://2012.igem.org/Team:Calgary/Project/FRED/Prototype">hardware and software platform</a> for a field-ready biosensor. Our system has also been <a href="https://2012.igem.org/Team:Calgary/Project/FRED/Modelling">mathematically modeled</a> in MATLAB to aid us in planning time courses for the experiments and the final prototype. When combined with the mechanical and biological containment mechanisms used in our system these genes create a novel and safe approach to biosensing in the oil sands and in many other potential applications.</p> | <p>With our electrochemical system functioning properly we can now hook up our reporter genes to promoters found in the <a href="https://2012.igem.org/Team:Calgary/Project/FRED/Detecting">transposon library</a> for a final detection system. We have also created a <a href="https://2012.igem.org/Team:Calgary/Project/FRED/Prototype">hardware and software platform</a> for a field-ready biosensor. Our system has also been <a href="https://2012.igem.org/Team:Calgary/Project/FRED/Modelling">mathematically modeled</a> in MATLAB to aid us in planning time courses for the experiments and the final prototype. When combined with the mechanical and biological containment mechanisms used in our system these genes create a novel and safe approach to biosensing in the oil sands and in many other potential applications.</p> | ||
Revision as of 18:54, 3 October 2012


Hello! iGEM Calgary's wiki functions best with Javascript enabled, especially for mobile devices. We recommend that you enable Javascript on your device for the best wiki-viewing experience. Thanks!
A Novel Electrochemical Reporting System

For FRED to be able to tell us about the toxins he's sensing we needed a good reporter system that could function in a wide array of environments. Unfortunately the traditional fluorescent or luminescent reporters have significant drawbacks that prevent them from being useful in a tailings environment that is murky and potentially anaerobic. Due to these limitations we decided to improve upon last year's single output electrochemical sensor using the lacZ gene to cleave a substrate into an easily detectable analyte. Our team has developed a novel system that utilizes three separate reporter genes to provide a triple-output electrochemical biosensor and can be used in a wide variety of applications. This system overcomes traditional reporters in that it is fast, accurate, and can function in turbid environments and even in the absence of oxygen!
How Does it Work?
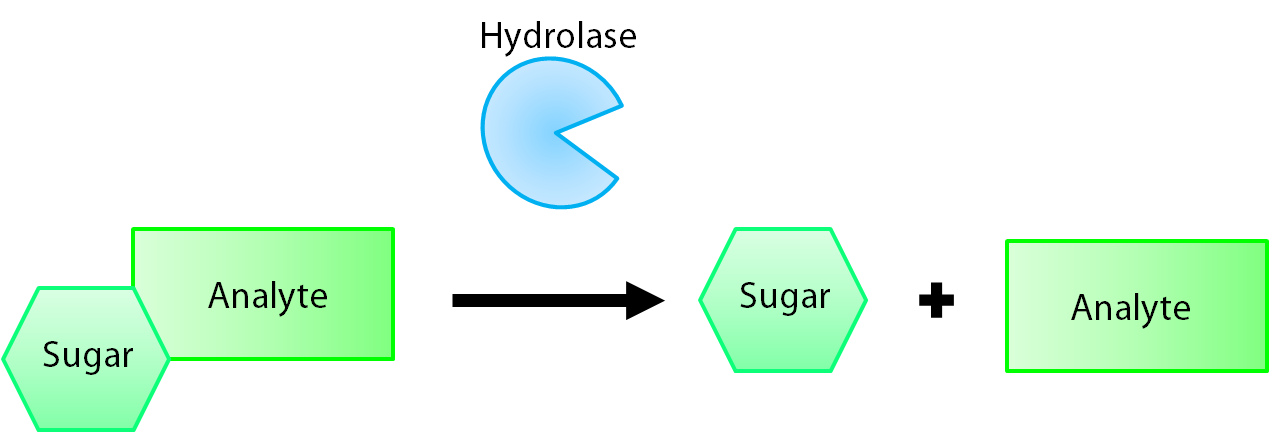
The enzymes encoded by our reporter genes are specific sugar hydrolases. This means that they target one kind of sugar and remove it from whatever compound they are attached to. We have chosen to use the sugars glucose, glucuronide, and galactose for our system. The genes responsible for their respective hydrolases are bglX, uidA, and lacZ. By having our electrochemical analyte conjugated to this sugar, when the hydrolase is expressed the sugar is cleaved from the analyte, allowing for it's electrochemical detection. A diagrammatic representation of this system is shown below in figure 2.
After the analyte is released we need to detect it. Electrochemistry is an excellent approach for this because of it's fast and quantitative nature. A voltage is applied between two electrodes compared to a reference electrode and the resulting current is measured. By changing the applied voltage to that of the oxidation voltage of one of our analytes, the increase in current due to it's oxidation when compared to an analyte free baseline is proportional to the amount of analyte present in the solution. This process happens so quickly that you can have an output value in a matter of seconds.
We used two different electrochemical techniques in our testing depending on what question the experiment was trying to answer. When we were characterizing the voltages at which our products oxidized we used cyclic voltammetry, which is where you apply a voltage and then slowly increase and decrease it over a designated sweep range. Any bumps in the graph are due to a reaction and can be standardized against baseline measurements. After the oxidation potential has been localized we can speed up our experiments by using potentiostatic runs. In this case, instead of sweeping the voltage we apply to the solution we hold it steady at the voltage that will oxidize our compound the moment it is released into the solution. Both of these techniques require the three electrodes in an electrolyte solution such as phosphate buffered saline and can routinely detect nanomolar concentrations of electrochemical analytes.
Genes, Chemicals, and Circuits
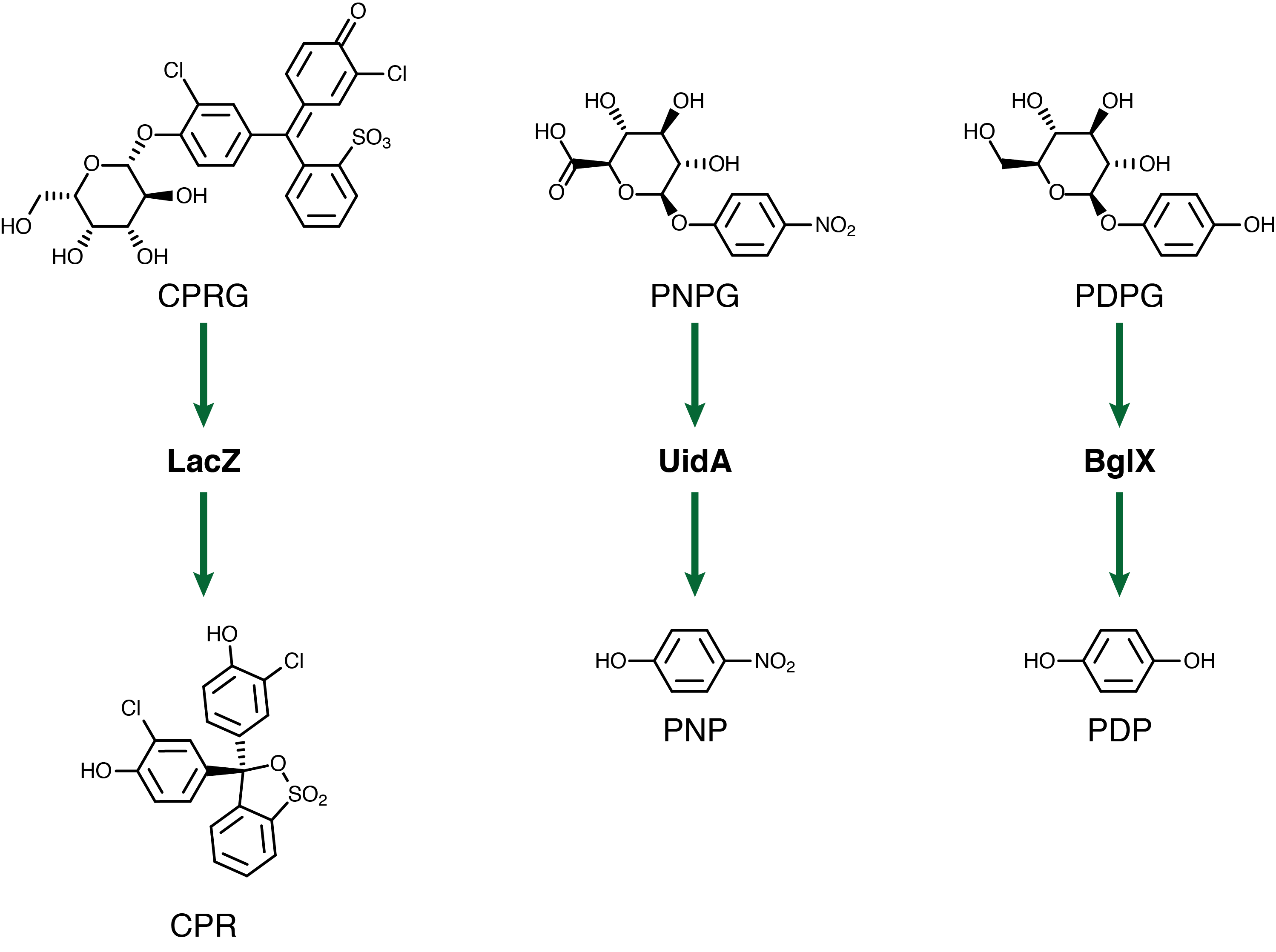
For our system to have a triplex output we need three separate genetic circuits with three analytes possessing unique oxidation potentials. If one chemical overlaps with another we could get false-positives of one chemical due to oxidation of another. To this end we have chosen to use chlorophenol red (CPR), para-diphenol (PDP), and para-nitrophenol (PNP). These compounds are conjugated with their sugars to form CPR-β-D-galactopyranoside (CPRG), PDP-β-D-glucopyranoside (PDPG), and PNP-β-D-glucuronide (PNPG). An easy way to tell the analytes from their sugar conjugates is the addition of the letter G to the acronym. These chemicals are summarized below in figure 3 along with the reporter genes used with each one.
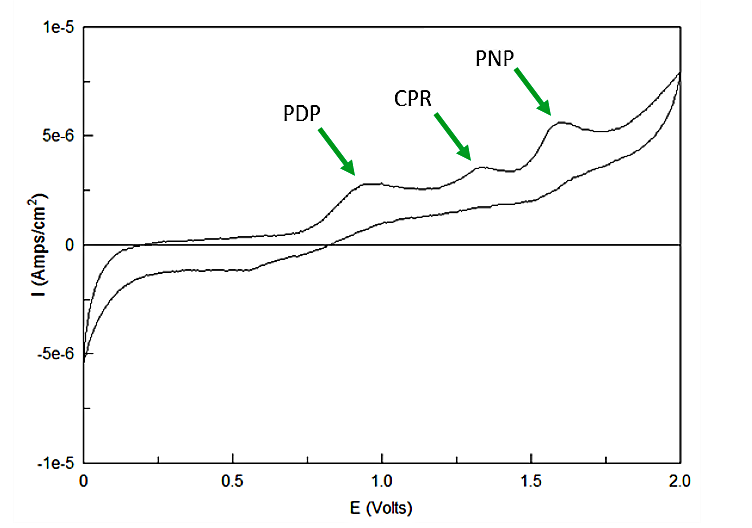
Out of the three sugar conjugates the only one that exhibits any electrochemical activity is PDPG, with it's oxidation potential at 0.6V vs. the reduction of hydrogen reference electrode (RHE). The three analytes have potentials at 0.825V for PDP, 1.325V for CPR, and 1.6V for PNP vs RHE. As none of these peaks overlap and no sugar conjugates interfere with their signals the three chemicals can be detected in the same solution. Figure 4 shows sensitive simultaneous detection of our three analytes with no background interference.
With the chemicals finalized we now needed to construct our circuits. As the lacZ gene under the control of the lacI promoter in the registry has a frameshift mutation rendering the enzyme nonfunctional, one of the constitutive lacZ hits from the transposon screen was used for initial characterization. The bglX and uidA genes were amplified from the E. coli genome using PCR and biobricked as BBa_K902004 and BBa_K902000 respectively. These genes were then constructed under the lacI promoter to allow for comparison testing.
Does it Work?
Yes! We have been able to show that we can detect the action of our hydrolase enzymes acting on the sugar-conjugated compounds to give us an electrochemical signal. This is shown below in figure 5.
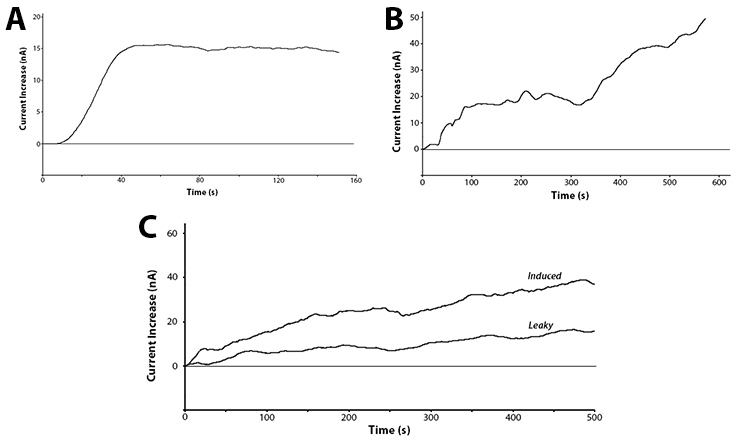
These graphs show two main points. The first being that we can successfully use hydrolase enzymes as reporters for gene expression with a sensitive output. As these reporters do not rely on having a colour or fluorescence output they can be used in turbid solutions. This removes one of the limitations of biosensors, potentially allowing this branch of biotechnology access to a new market.
The second interesting conclusion that can be drawn for part C of figure 5 is the leakiness of the BBa_R0010 promoter. The bacteria were induced at time zero and a clear increase is seen almost immediately, but the current does still increase over time for the uninduced test. The leaky expression of the genes downstream of this promoter could be detrimental in situations such as toxic gene expression or time dependent events.
What Next?
As we have shown in figure 5C, we can accurately and extremely quickly detect the level of expression of a promoter. The ability to do this in a matter of minutes allows multitudes of promoters to be characterized in an extremely short span of time. Being able to quantitatively detect extremely low expression also gives credit to the idea that electrochemical reporters are a usable standard for detecting levels of promoter expression. Having one reporter constitutively expressed as a population counter, a second as a negative control, and the third as the promoter reporter would create a robust procedure with built in controls for measuring promoter expression.
With our electrochemical system functioning properly we can now hook up our reporter genes to promoters found in the transposon library for a final detection system. We have also created a hardware and software platform for a field-ready biosensor. Our system has also been mathematically modeled in MATLAB to aid us in planning time courses for the experiments and the final prototype. When combined with the mechanical and biological containment mechanisms used in our system these genes create a novel and safe approach to biosensing in the oil sands and in many other potential applications.
 "
"