Team:Calgary/Project/OSCAR/CatecholDegradation
From 2012.igem.org
| (38 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Team:Calgary/TemplateProjectBlue| | {{Team:Calgary/TemplateProjectBlue| | ||
| - | TITLE= | + | TITLE=Decatecholization| |
CONTENT=<html> | CONTENT=<html> | ||
<img src="https://static.igem.org/mediawiki/2012/1/1c/UCalgary2012_OSCAR_Catechol_Low-Res.png" style="float: right; padding: 10px;"></img> | <img src="https://static.igem.org/mediawiki/2012/1/1c/UCalgary2012_OSCAR_Catechol_Low-Res.png" style="float: right; padding: 10px;"></img> | ||
| - | |||
| - | |||
| + | <p>Catechol is a toxic compound found in tailings ponds that is a by-product of polyaromatic hydrocarbon metabolism (Vaillancourt <i>et al.</i>, 2006, Schweigert <i>et al.</i>, 2001)). The chemical properties of catechol allow it to react with biomolecules, causing cellular damage including DNA damage, enzyme inactivation and membrane uncoupling (Schweigert <i>et al.</i>, 2001). </p> | ||
| + | <p> | ||
| + | Catechol is characterized as having a benzene ring with two hydroxyl groups at the 2,3 position. It can be converted to 2-hydroxymuconic acid by the enzyme catechol 2,3-dioxygenase, encoded by the <i>xylE</i> gene on the Tol plasmid of <i>Pseudomonas putida</i> (Nakai <i>et al.</i>, 1983).</p> | ||
| + | |||
| + | <p> | ||
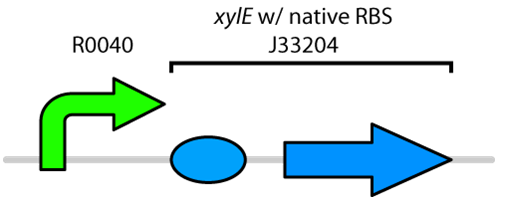
| + | Currently the registry has two BioBricks available of <i>xylE</i>. One contained <i>xylE</i> with its native ribosome-binding site (<a href=http://partsregistry.org/Part:BBa_J33204>BBa_J33204</a>), while the other part contained <i>xylE</i> under the glucose-repressible promoter <i>cstA </i>(<a href=http://partsregistry.org/Part:BBa_K118021>BBa_K118021</a>). Given that <i>E. coli</i> is grown in the presence of glucose, we designed a new construct to keep <i>xylE</i> expressed by using the <i>tetR</i> promoter (<a href= http://partsregistry.org/Part:BBa_R0040>BBa_R0040</a>).</p> | ||
| + | |||
| + | </html>[[File:UCalgary2010_R0040-XylE.png|400px|thumb|Figure 1: BioBrick genetic circuit for catechol degradation showing <i>xylE</i> under the ''tetR'' promoter|center]]<html> | ||
<h3></h3> | <h3></h3> | ||
| - | |||
| - | |||
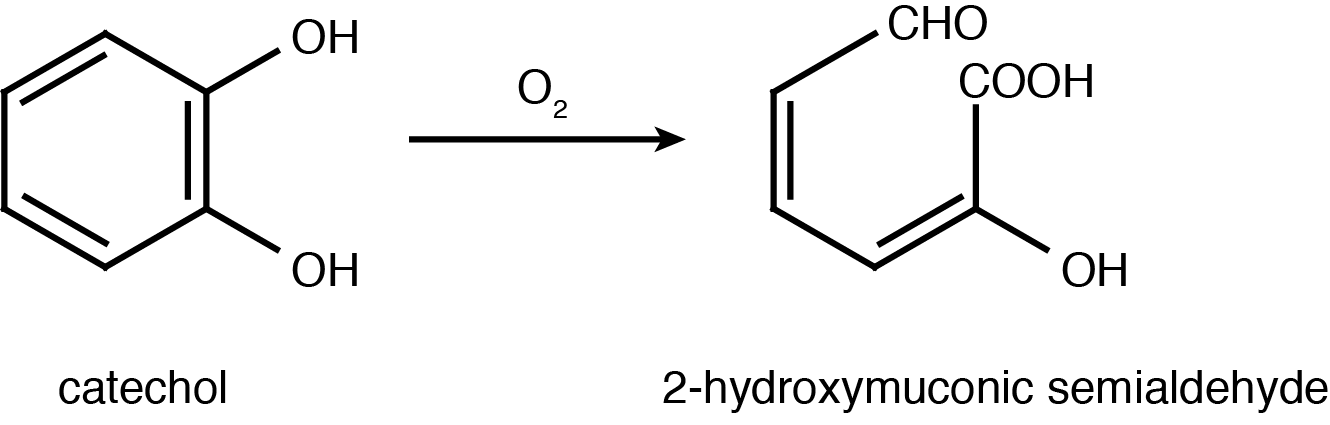
| - | <p>Catechol 2,3-dioxygenase is an extradiol dioxygenase which cleaves catechol adjacent to the two hydroxyl groups. When this occurs 2-hydroxymuconate semialdehyde is produced, which is yellow in colour. This change in colour allows for visual assay to assess the activity of < | + | <p>Catechol 2,3-dioxygenase is an extradiol dioxygenase which cleaves catechol adjacent to the two hydroxyl groups. When this occurs 2-hydroxymuconate semialdehyde is produced, which is yellow in colour. This change in colour allows for visual assay to assess the activity of XylE.</p> |
| + | |||
| + | </html>[[File:UCalgary2012_Catechol_to_2-HMS.PNG|400px|thumb|Figure 2: Catechol 2,3-dioxygenase (XylE) converts catechol to 2-hydroxymuconate semialdehyde in the presence of oxygen. Adapted from Shu <i>et al</i>., 1995.|center]]<html> | ||
| + | |||
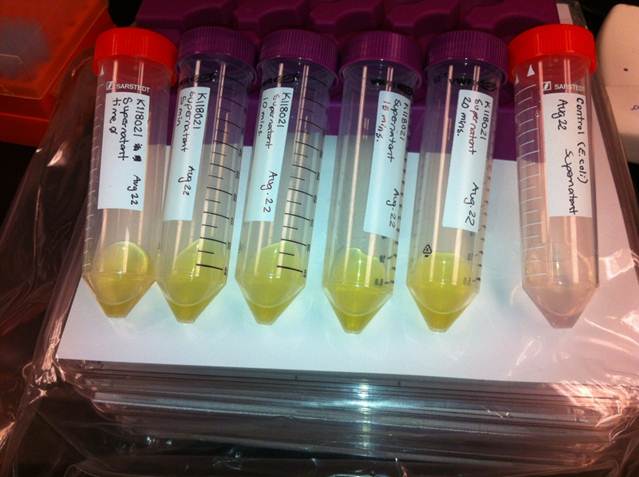
| + | <p>The visual assays were performed with <i>E. coli</i> cells transformed with (<a href=http://partsregistry.org/Part:BBa_K118021>BBa_K118021</a>) as well as with <i>E. coli</i> cells transformed with the newly constructed part (<a href=http://partsregistry.org/Part:BBa_K902048 >BBa_K902048</a>) by bringing the supernatant of an overnight culture to a concentration of 0.1 M of catechol. When the part (<a href=http://partsregistry.org/Part:BBa_K118021>BBa_K118021</a>) was used, the pellet was first washed in M9-MM and centrifuged before catechol was added to the supernatant. This was necessary to avoid the glucose in the LB from repressing the cstA promoter (<a href=http://partsregistry.org/Part:BBa_K118011>BBa_K118011</a>). Catechol was added to the supernatant because the reaction takes place outside of the cell. Within minutes of the addition of catechol to the supernatant, the solution turned from the pale yellow of LB to a bright yellow. This was indicative that catechol was breaking down into 2-hydroxymuconate semialdehyde, which was exactly what we expected! This assay was completed by following the protocol written by the 2008 Edinburgh iGEM team.</p> | ||
| + | |||
| + | </html>[[File:UCalgary2012_Catechol_assay.jpg|500px|thumb|Figure 3: Results of the catechol visual assay using ''xylE'' [http://partsregistry.org/Part:BBa_K118021 BBa_K118021]. Cultures were grown overnight in LB and the pellets were washed with M9-MM at various times (From left to right: 0 min, 5 min, 10 min, 15 min, and 20 min.). Cells were then spun down and catechol was added to the supernatant to 0.1 M. The amount of time didn't affect the colour change in the cultures containing the <i>xylE</i> gene. The far right tube has <i>E. coli</i> cells without the <i>xylE</i> gene as a negative control and the supernatant remained clear when the catechol was added. |center]]<html> | ||
| + | |||
| + | <a name="Catechol"></a><h2> Converting Catechol into hydrocarbons? </h2> | ||
| + | <p>After verifying that we could in fact degrade catechol into 2-hydroxymuconate semialdehyde using our <i>xylE</i> construct (<a href=http://partsregistry.org/Part:BBa_J33204>BBa_J33204</a>), we wondered if we could take this any further. What if we could convert this by-product into hydrocarbons too? As catechol is the breakdown product of a number of different degradation pathways in bacteria, this could be particularly useful.</p> | ||
| + | |||
| + | <p>Since 2-hydroxymuconate semialdehyde can be further metabolized to pyruvate and acetaldehyde (Harayama et al., 1987), it seemed possible that these products could be routed into the fatty acid biosynthesis pathway and converted to alkanes using the PetroBrick or the OleT enzyme. Given that the catechol 2,3-dioxygenase reaction is extracellular, it creates a possible scenario in which cells with the <i>xylE</i> construct could be co-cultured with Petrobrick-containing cells to cooperatively metabolize catechol into hydrocarbons. </p> | ||
| + | |||
| + | <p> In order to test this, we followed this <a href=https://2012.igem.org/Team:Calgary/Notebook/Protocols/decatecholization>protocol</a>, where we co-cultured cells expressing our <i>xylE</i> construct with either <i>E. coli</i> cells expressing the PetroBrick, or <i>Jeotgalicoccus</i> sp. ATCC 8456 cells expressing <i>oleT</i> in the presence of catechol. | ||
| + | |||
| + | |||
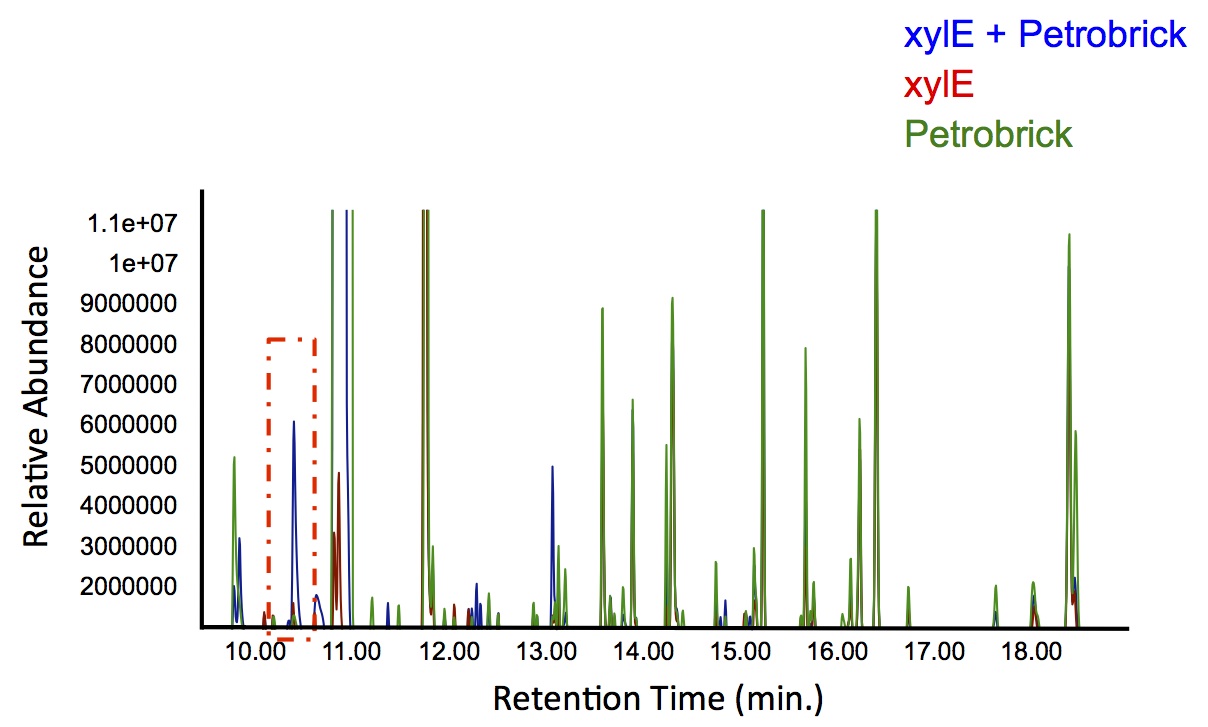
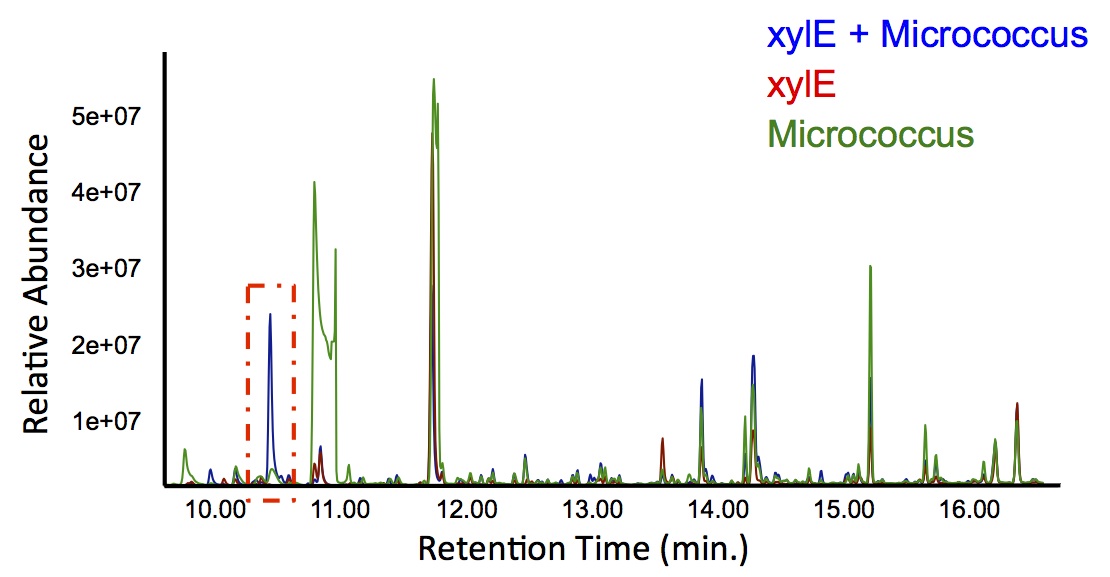
| + | </html>[[File:Calgary PetrobrickCatechol.jpg|600px|thumb|centre|Figure 4: Gas chromatograph of catechol degradation assay using the PetroBrick. While there is limited differences between <i>xylE</i> incubated with and without the PetroBrick, there was one peak with a retention time of 10.5 min which was dramatically increased in the co-culture.]]<html> | ||
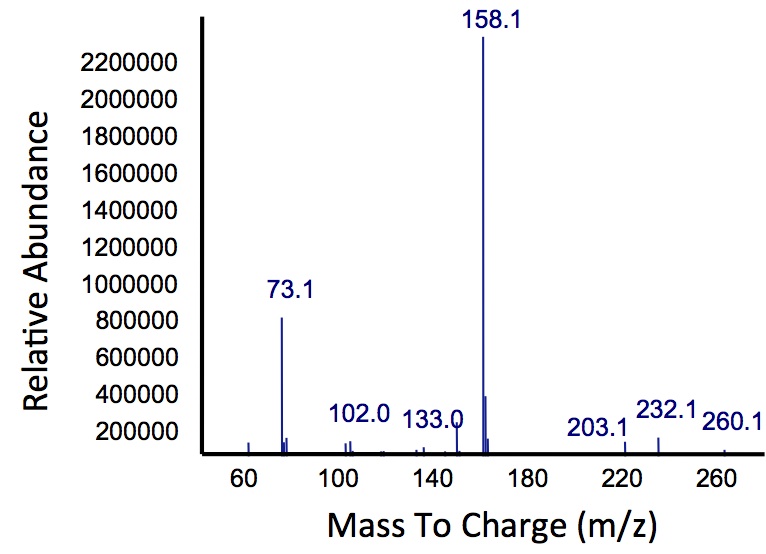
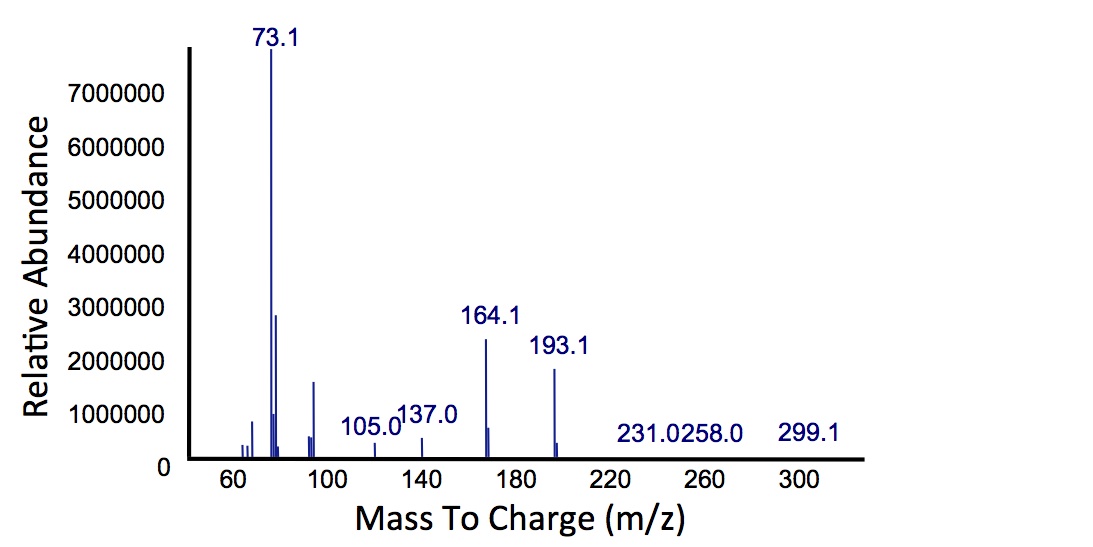
| - | </html>[[File: | + | </html>[[File:Calgary MSCatecholPetroPeak.jpg|450px|thumb|centre|Figure 5: Mass spectrum of the Petrobrick/<i>xylE</i> co-culture retention peak at 10.5 min as shown in Figure 4. While the identity of this compound is currently unknown, there are changes occuring to some of the catechol breakdown products.]]<html> |
| - | + | </html>[[File:Calgary CatechololeTGC.jpg|600px|thumb|centre|Figure 6: Gas chromatograph of catechol degradation assay using <i>Jeotgalicoccus</i> sp. ATCC 8456 a species of bacteria that converts fatty acids into alkenes. This identified a similar peak change in the PetroBrick with a retention time of 10.5 min as shown in Figure 4. This provides additional support that the PetroBrick and this organism can further degrade catechol into breakdown products.]]<html> | |
| - | </html>[[File: | + | </html>[[File:Calgary CatecholMSoleT.jpg|600px|thumb|centre|Figure 7: Mass spectrum of <i>Jeotgalicoccus</i> sp. ATCC 8456/<i>xylE</i> co-culture retention peak at 10.5 min as shown in Figure 6. This peak is similar to the peak from the Petrobrick/<i>xylE</i> co-culture, suggesting the breakdown product for both of these cultures is modified catechol from <i>xylE</i>. The identification of this compound is ongoing.]]<html> |
| + | <p> Based on our GC-MS results, we were able to show the appearance of a new peak when cells expressing <i>xylE</i> and the PetroBrick were co-cultured. Although we don't know the exact identity of this peak, it is distinct form our control. It resembles standards that contain a toluene substituent suggesting that this compound maybe similar in structure. This structure is also made possible by the fact that the catechol degradation product is capable of forming polymers and potentially ring type structures. It may very well be that these ring structures are being further processed by the Petrobrick into useful products, which we hope to identify through GC-MS/MS in the future. Interestingly, a similar peak appeared when cells expressing our <i>xylE</i> construct were co-cultured with <i>Jeotgalicoccus</i> sp. ATCC 8456 cells. This suggests that although we don't know the exact identity of this new peak, it is likely that it may be in fact a further breakdown product of catechol. This is a very promising result, as it suggests that in addition to converting naphthenic acids into hydrocarbons, we may also be able to break down catechol, one of the other major toxic components in tailings ponds.</p> | ||
Latest revision as of 02:24, 27 October 2012


Hello! iGEM Calgary's wiki functions best with Javascript enabled, especially for mobile devices. We recommend that you enable Javascript on your device for the best wiki-viewing experience. Thanks!
Decatecholization

Catechol is a toxic compound found in tailings ponds that is a by-product of polyaromatic hydrocarbon metabolism (Vaillancourt et al., 2006, Schweigert et al., 2001)). The chemical properties of catechol allow it to react with biomolecules, causing cellular damage including DNA damage, enzyme inactivation and membrane uncoupling (Schweigert et al., 2001).
Catechol is characterized as having a benzene ring with two hydroxyl groups at the 2,3 position. It can be converted to 2-hydroxymuconic acid by the enzyme catechol 2,3-dioxygenase, encoded by the xylE gene on the Tol plasmid of Pseudomonas putida (Nakai et al., 1983).
Currently the registry has two BioBricks available of xylE. One contained xylE with its native ribosome-binding site (BBa_J33204), while the other part contained xylE under the glucose-repressible promoter cstA (BBa_K118021). Given that E. coli is grown in the presence of glucose, we designed a new construct to keep xylE expressed by using the tetR promoter (BBa_R0040).
Catechol 2,3-dioxygenase is an extradiol dioxygenase which cleaves catechol adjacent to the two hydroxyl groups. When this occurs 2-hydroxymuconate semialdehyde is produced, which is yellow in colour. This change in colour allows for visual assay to assess the activity of XylE.
The visual assays were performed with E. coli cells transformed with (BBa_K118021) as well as with E. coli cells transformed with the newly constructed part (BBa_K902048) by bringing the supernatant of an overnight culture to a concentration of 0.1 M of catechol. When the part (BBa_K118021) was used, the pellet was first washed in M9-MM and centrifuged before catechol was added to the supernatant. This was necessary to avoid the glucose in the LB from repressing the cstA promoter (BBa_K118011). Catechol was added to the supernatant because the reaction takes place outside of the cell. Within minutes of the addition of catechol to the supernatant, the solution turned from the pale yellow of LB to a bright yellow. This was indicative that catechol was breaking down into 2-hydroxymuconate semialdehyde, which was exactly what we expected! This assay was completed by following the protocol written by the 2008 Edinburgh iGEM team.
Converting Catechol into hydrocarbons?
After verifying that we could in fact degrade catechol into 2-hydroxymuconate semialdehyde using our xylE construct (BBa_J33204), we wondered if we could take this any further. What if we could convert this by-product into hydrocarbons too? As catechol is the breakdown product of a number of different degradation pathways in bacteria, this could be particularly useful.
Since 2-hydroxymuconate semialdehyde can be further metabolized to pyruvate and acetaldehyde (Harayama et al., 1987), it seemed possible that these products could be routed into the fatty acid biosynthesis pathway and converted to alkanes using the PetroBrick or the OleT enzyme. Given that the catechol 2,3-dioxygenase reaction is extracellular, it creates a possible scenario in which cells with the xylE construct could be co-cultured with Petrobrick-containing cells to cooperatively metabolize catechol into hydrocarbons.
In order to test this, we followed this protocol, where we co-cultured cells expressing our xylE construct with either E. coli cells expressing the PetroBrick, or Jeotgalicoccus sp. ATCC 8456 cells expressing oleT in the presence of catechol.
Based on our GC-MS results, we were able to show the appearance of a new peak when cells expressing xylE and the PetroBrick were co-cultured. Although we don't know the exact identity of this peak, it is distinct form our control. It resembles standards that contain a toluene substituent suggesting that this compound maybe similar in structure. This structure is also made possible by the fact that the catechol degradation product is capable of forming polymers and potentially ring type structures. It may very well be that these ring structures are being further processed by the Petrobrick into useful products, which we hope to identify through GC-MS/MS in the future. Interestingly, a similar peak appeared when cells expressing our xylE construct were co-cultured with Jeotgalicoccus sp. ATCC 8456 cells. This suggests that although we don't know the exact identity of this new peak, it is likely that it may be in fact a further breakdown product of catechol. This is a very promising result, as it suggests that in addition to converting naphthenic acids into hydrocarbons, we may also be able to break down catechol, one of the other major toxic components in tailings ponds.
 "
"