Team:Calgary/Project/FRED/Detecting
From 2012.igem.org
| Line 5: | Line 5: | ||
<html> | <html> | ||
| - | <img src="https://static.igem.org/mediawiki/2012/5/52/UCalgary2012_FRED_Detecting.png" style="float: right; padding: 10px; height: | + | <img src="https://static.igem.org/mediawiki/2012/5/52/UCalgary2012_FRED_Detecting.png" style="float: right; padding: 10px; height: 180px;"></img> |
<p align="justify"> | <p align="justify"> | ||
This year, our team wanted to identify a novel responsive element capable of detecting and quantifying tailings ponds toxins (e.g. naphthenic acids/NAs) in solution. While numerous studies have begun to identify species of bacteria capable of surviving and sensing a variety of toxic compounds (e.g. naphthenic acids/NAs), the degradation pathways have not yet been fully characterized. Therefore, we needed to design and implement novel approaches to efficiently isolate the genetic elements that detect and potentially lead to the breakdown of these toxins. | This year, our team wanted to identify a novel responsive element capable of detecting and quantifying tailings ponds toxins (e.g. naphthenic acids/NAs) in solution. While numerous studies have begun to identify species of bacteria capable of surviving and sensing a variety of toxic compounds (e.g. naphthenic acids/NAs), the degradation pathways have not yet been fully characterized. Therefore, we needed to design and implement novel approaches to efficiently isolate the genetic elements that detect and potentially lead to the breakdown of these toxins. | ||
Revision as of 21:22, 3 October 2012


Hello! iGEM Calgary's wiki functions best with Javascript enabled, especially for mobile devices. We recommend that you enable Javascript on your device for the best wiki-viewing experience. Thanks!
A Transposon-Mediated Mutant Library for Toxin Detection

This year, our team wanted to identify a novel responsive element capable of detecting and quantifying tailings ponds toxins (e.g. naphthenic acids/NAs) in solution. While numerous studies have begun to identify species of bacteria capable of surviving and sensing a variety of toxic compounds (e.g. naphthenic acids/NAs), the degradation pathways have not yet been fully characterized. Therefore, we needed to design and implement novel approaches to efficiently isolate the genetic elements that detect and potentially lead to the breakdown of these toxins.
Transposons: What, How, Why?
The transposable element (TE), Tn5, is a conservative transposon that is able to insert a segment of genes bordered by specific 19bp insertion sequences (IS) from one part of the genome (e.g. plasmid vector) randomly to another location, such as the chromosome (Reznikoff, 2008). The transposition event is catalyzed by a transposase enzyme encoded by Tnp gene included in the TE. Using the appropriate selective pressure, the insertion can be maintained permanently in the genome.
By inserting a vector construct containing the TE with selectable markers (such as tetracyclin resistance and lacZ) into an organism with a desirable phenotype, we can find out what genetic elements (e.g. genes and promoters) are responsible for that particular function. This can happen via a random insertion of a TE containing a promoterless reporter gene downstream of promoter elements that creates a transcriptional fusion, providing activity in response to specific environmental stimuli. Using a bipartite-mating (conjugation) method to transfer the TE vector into the organism of choice is an efficient at creating the massive number of mutants required.
Due to the complexity of biological systems, our team focused our efforts on identifying a system for identification of promoter elements that respond specifically in the presence of environmental stimuli. Our hypothesis requires that the organisms we use respond specifically to particular toxins and result in specific upregulation of metabolic genes with little background effect in the cell. We recognize that any number of biological molecules within a NA-degrading organism may play role in the sensing and modification of NAs, such as enzymes, transcription factors, and even RNA elements (e.g. riboswitches). However, the identification of a promoter sequence takes us further in that we can better understand the mechanism of NA degradation by elucidating the genes involved.
Naphthenic Acid Degrading Organism Used
Pseudomonads have been isolated from oil sands tailings ponds and shown to biodegrade model and tailings-associated NAs (Ramos-Padrón et al. 2010; Herman et al., 1994; Del Rio et al., 2006; Gieg & Whitby, unpublished, 2012). Since they can degrade NAs, this suggests that there is a system that detects and is up-regulated specifically in response to NAs. We wanted to use a commercially available strain of Pseudomonas fluorescens characterized for a response to model NAs (model single- and double-ringed compounds) and NAs isolated from tailings pond water (TPW). The P. fluorescens pf-5 strain is reported to survive in and degrade a commercial mixture of naphthenic acids (Acros) (Gieg & Whitby unpublished, 2012). Moreover, sequencing data is available for this strain with annotations (Pseudomonas Genome Database V2, http://pseudomonas.com/). This allows us to use sequencing data from the mutants and identify where in the genome the TE insertion occured, and what genes (if present) are located downstream of it.
Method Design
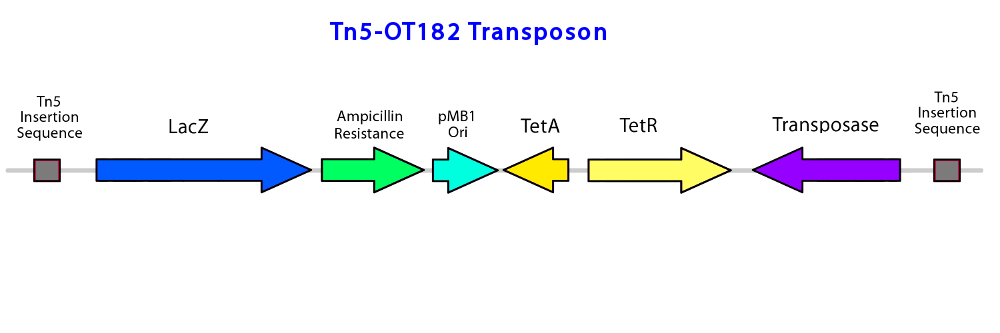
To construct the promoter library, a pOT182 vector construct (containing a IR-lacZ-Amp-pMB1ori-TetA-TetR-Tnp-IR transposable element) is introduced into commercially purchased E. coli SM10 donor strain.
The plasmid is engineered in a vector containing a RP4 mob conjugation region, and a p15A origin of replication (ori). The IR-lacZ-Amp-pMB1ori-TetA-TetR-Tnp-IR construct is transferred from the E. coli donor strain to the recipient P. fluorescens pf-5 using bipartite mating via conjugation (enabled by the RP4 mob region). A random genomic library of transposon insertions is created in P. fluorescens, and selected by isolating the recipients on Pseudomonas Isolation Agar/PIA (the antibiotic Irgasan in this medium kills all other bateria, and only allows the survival of Pseudomonas spp.) and the recipient cells that have a genomic TE insertion using tetracyclin (conjugated plasmids cannot replicate in Pseudomonas, and thus the Pseudomonas that survived must have a TE insertion in the genomic DNA). If a promoter element is fused upstream of the TE construct, then promoter activation will turn on the expression of lacZ, which can be detected by the degradation of a colorless compound, X-Gal, to an insoluble blue pigment product (an indoxyl compound) (reference). If the fused promoter is activated in response to NAs, then lacZ will be produced in response to NAs. Desirable mutants are selected by plating the conjugated bacteria onto an agar plate containing PIA, tetracyclin (at a concentration that wildtype Pseudomonas fluorescens pf-5 is sensitive to), a NA mixture (Acros), and X-Gal. Mutants are sensitive to NAs will appear as blue colonies on the plate.
Promoters identified by the transposon mutagenesis strategy are characterized for their roles in the response to NA exposure with dose response curves, and compared to general stress-inducing agents (e.g. H2O2) and compounds such as fatty acids to ensure the specificity of the response. These measurements help to determine thresholds of detection, robustness of the signal, and specificity of response. The dose response curves will also assess the usefulness of correlating the concentration of NA to the level of response, and the possibility of measuring NA concentrations in a sample, rather than simply by presence/absence.
Last, self-cloning techniques are used to identify the upstream and downstream sequences from the TE insertion (reference). IR-lacZ-Amp-pMB1ori-TetA-TetR-Tnp-IR is a self-cloning construct because it contains all the elements required for plasmid replication (i.e. origin of replication) and selection (Tet resistance). Genomic DNA from the desirable mutant is isolated, and restriction digested with either BglII or XbaI, which are restriction enzymes that do not cut within the transposon but numerous times within the genome. The resulting fragments may contain the TE construct with flanking sequences. The genomic fragments are circularized by self-ligation and transformed into E. coli Top10, and plated on LB agar with tetracyclin. The transformed cells now have plasmids containing the TE construct with the upstream and downstream flanking sequencing connected by the restriction site (of BglII or XbaI). Plasmids isolated from the transformed cells are used for sequencing. Sequencing primers are designed against the 19 bp recognition sequence in the TE to sequence outward from the transposon.
For a detailed protocol, please consult our methods section.
Results
Detection by Mutant Pseudomonas fluorescens Pf-5
After mating experiments and plating on selective media (pseudomonas isolation agar, with tetracycline and naphthenic acids), 24 responsive colonies were found. Screens were conducted on these blue colonies found on selective plates comparing a response in LB and LB with 100 mg/L naphthenic acids. When results were observed it was found that 4 colonies showed clear differential regulation in response to naphthenic acids: 66-1, 66-2, 170-1, and 199-1. Therefore, these colonies will be used in further screening to test the specificity of the response.
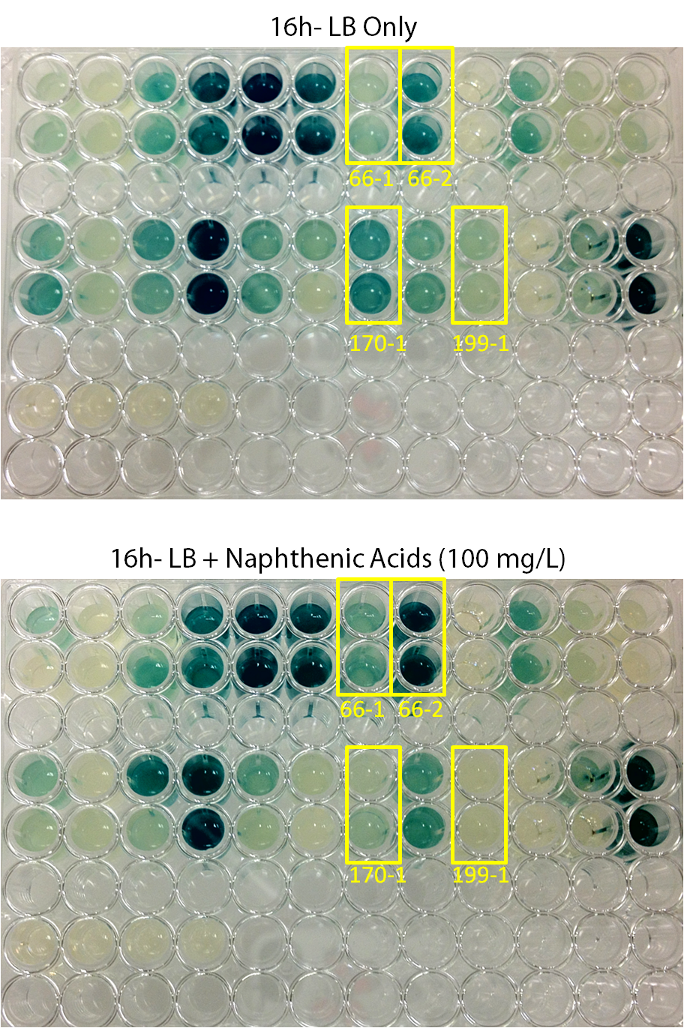
Screens involving the use of different toxins at environmentally relevant concentrations were performed to determine if the sensing response was specific to naphthenic acids, or if a sensory response to general toxins had been found. In addition, hydrogen peroxide was used in one of the media samples in order to attempt to rule out a general stress response by the cell.
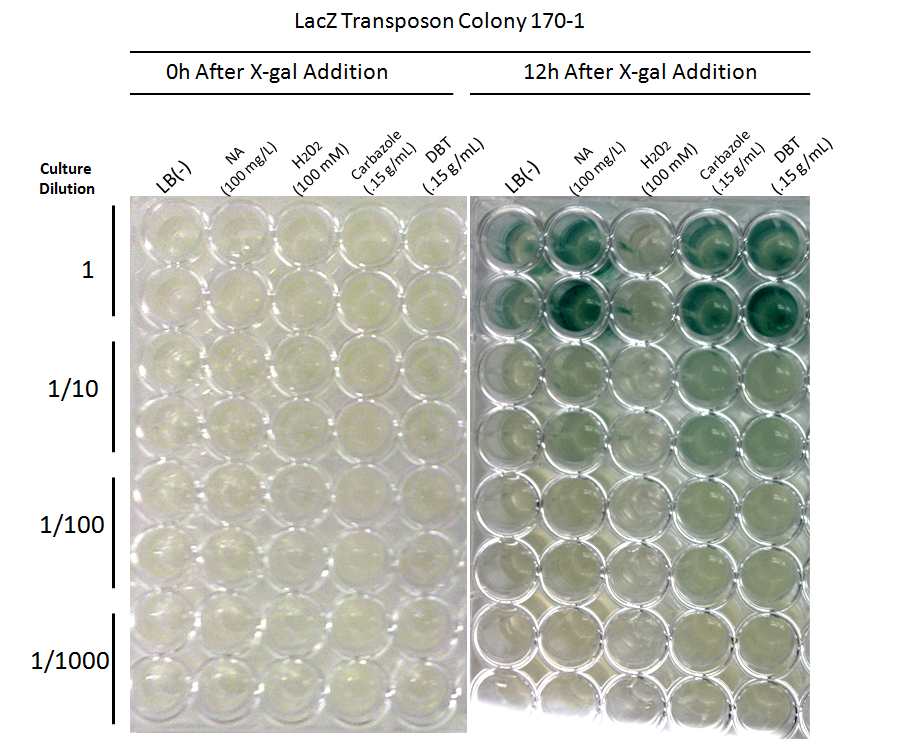
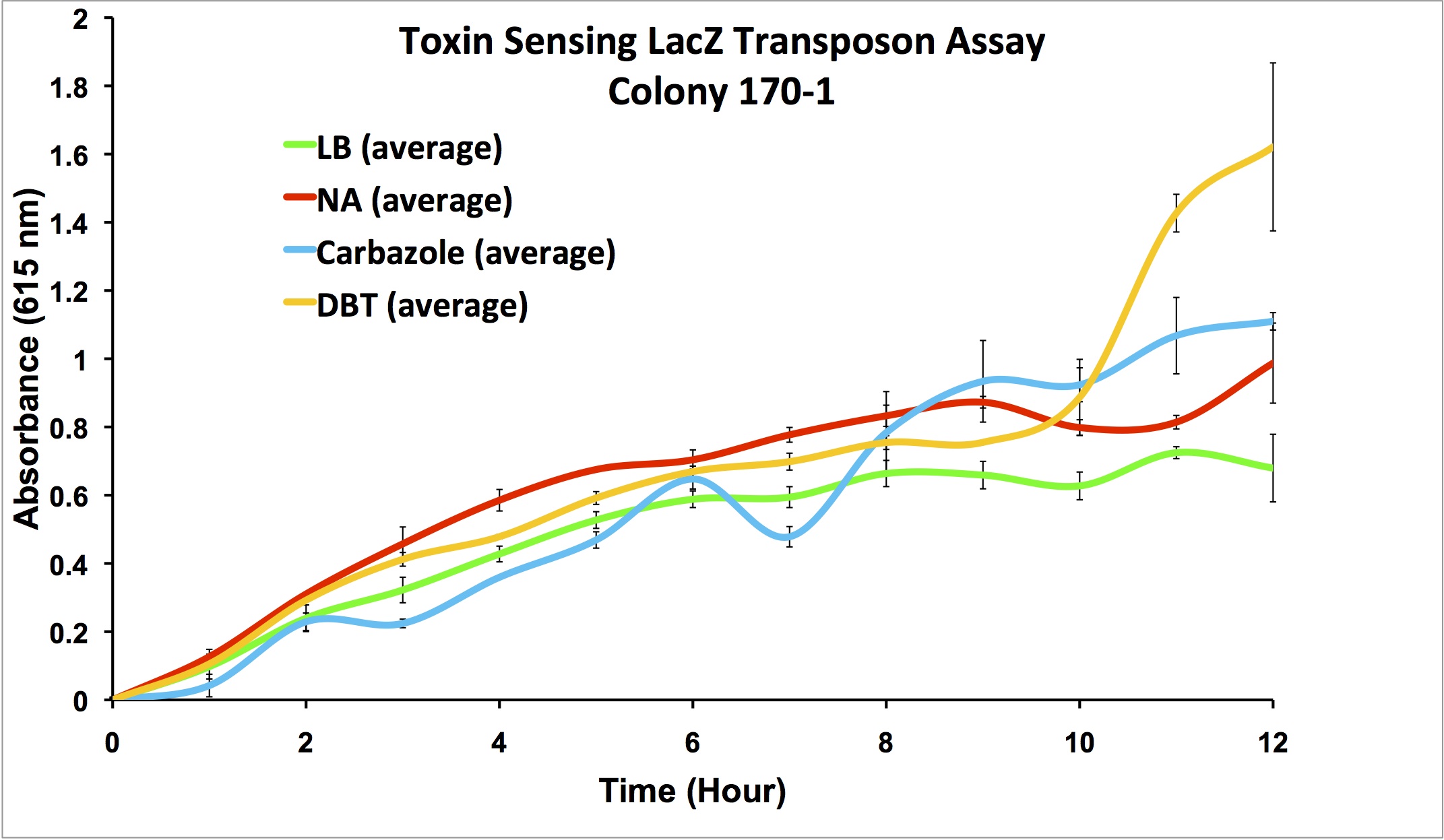

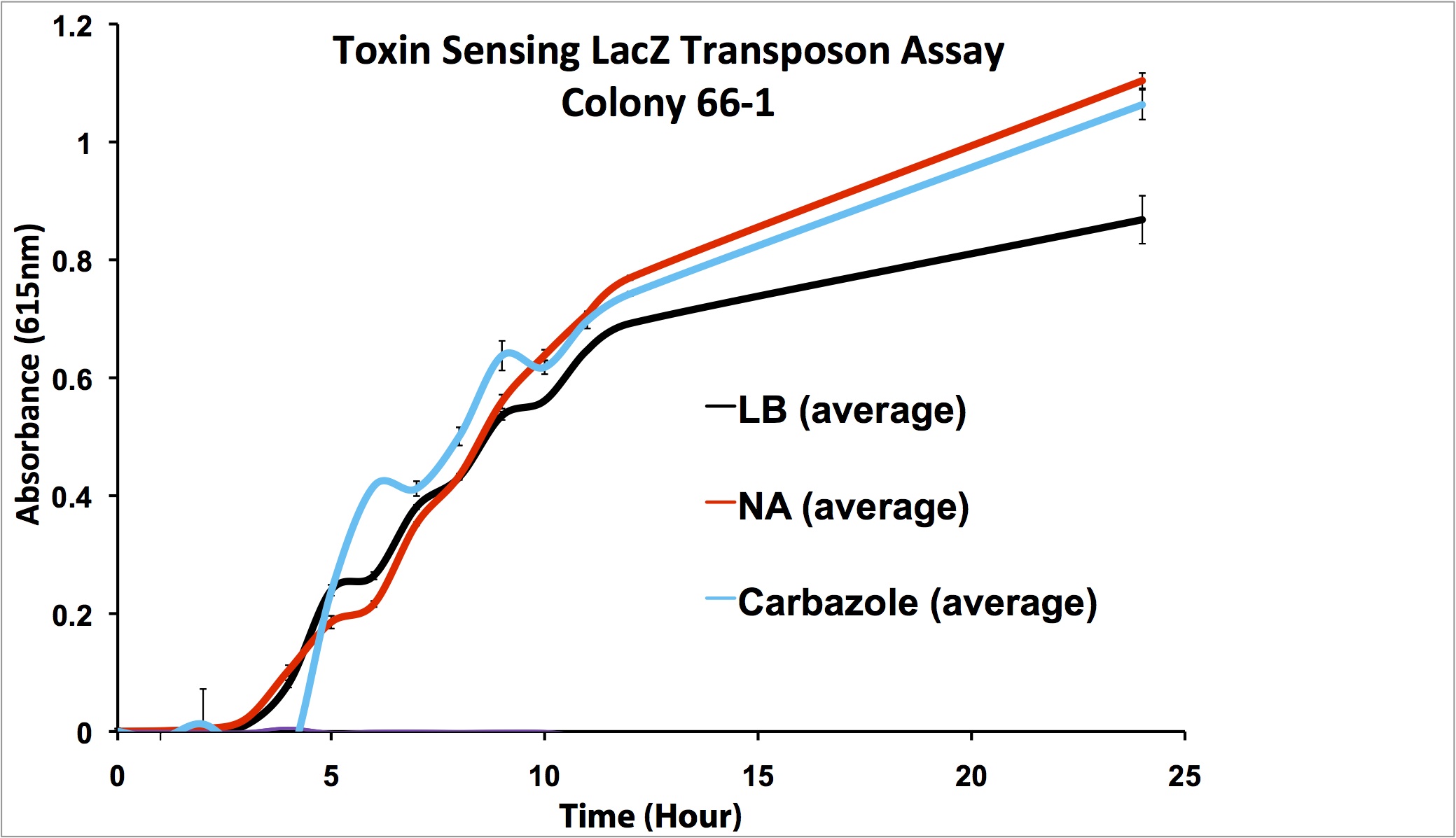
Promoter Constructs Isolated
In order to determine which genes the transposon has inserted into, the self-cloning properties of the transposon were utilized. By digesting the genome, religating, and transforming E. coli, plasmids containing the transposon and flanking gene sequences were isolated. We are still awaiting the sequencing results for these.
 "
"