Team:Calgary/Project/HumanPractices/Killswitch
From 2012.igem.org
| Line 26: | Line 26: | ||
<h3>Nuclease assay to evaluate the nucleases present in the registry (BglII and BamHI):</h3> | <h3>Nuclease assay to evaluate the nucleases present in the registry (BglII and BamHI):</h3> | ||
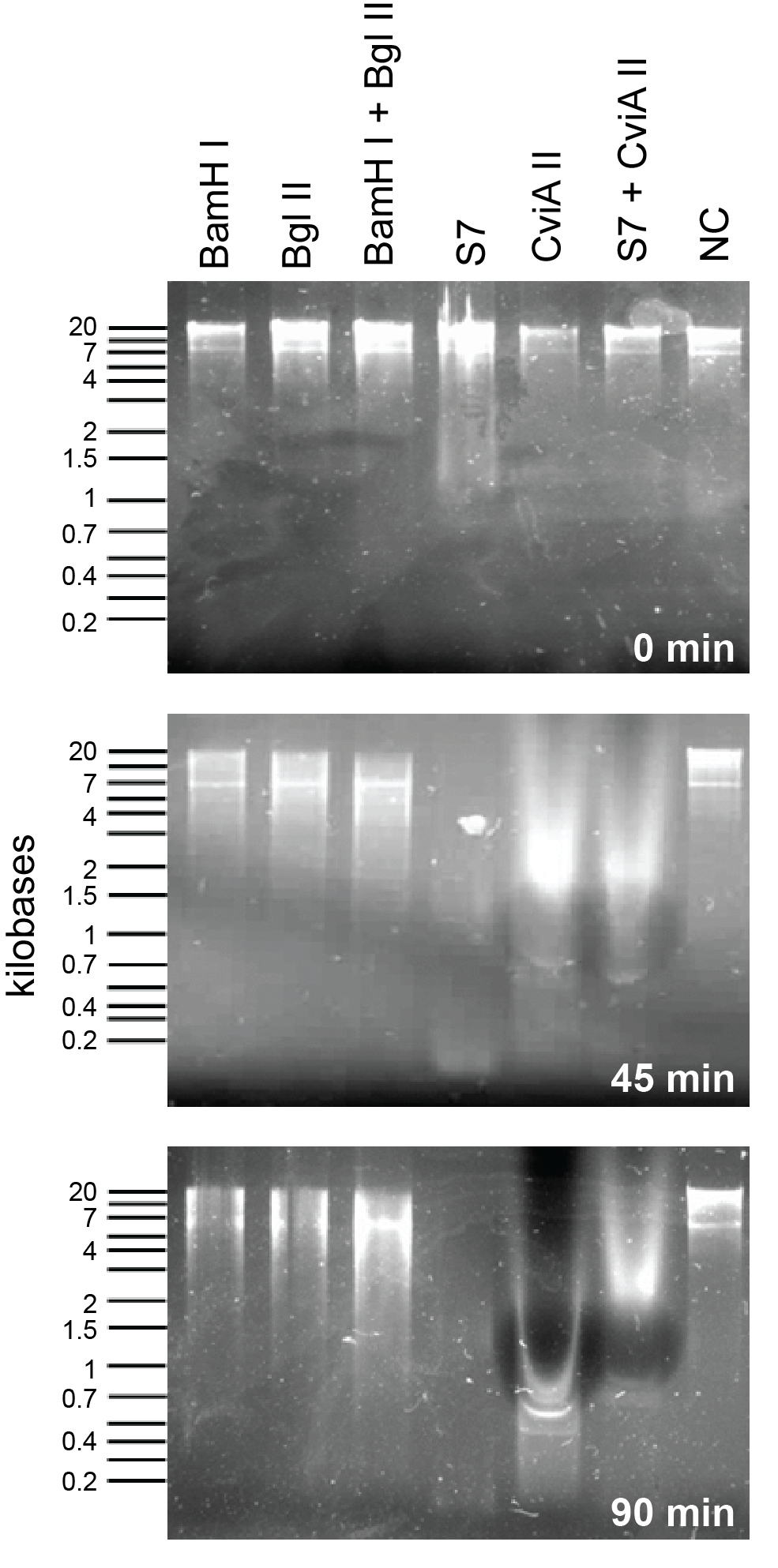
<P> To compare S7 and CviAII to the nucleases already present in the registry we did a nuclease assay with commercially available enzymes from New England Biolabs. To see detailed protocol please <link> see here</link> | <P> To compare S7 and CviAII to the nucleases already present in the registry we did a nuclease assay with commercially available enzymes from New England Biolabs. To see detailed protocol please <link> see here</link> | ||
| - | </html>[[File:UCalgary2012 RE-S7&CviaII.png|thumb|300px| | + | </html>[[File:UCalgary2012 RE-S7&CviaII.png|thumb|300px|left|Figure X: ]]<html> |
<h2>Regulation of our kill genes:</h2> | <h2>Regulation of our kill genes:</h2> | ||
Revision as of 07:18, 28 September 2012


Hello! iGEM Calgary's wiki functions best with Javascript enabled, especially for mobile devices. We recommend that you enable Javascript on your device for the best wiki-viewing experience. Thanks!
A Killswitch for Increased Security
Purpose:
Synthetic biology entails designing an organism to do a specific task. This involves genetic manipulation of the bugs and requires scientists to provide the bacteria with a selective advantage such as an antibiotic cassette which forces the bacteria to keep the gene of interest inside the cell. With such manipulation comes a valid “risk of accidental release” (Tucker and Zilinkas, 2006). Attempts have been made to address the concern regarding "accidental release". Some of these attempts include designing of lab strains, designing auxotrophes which cannot synthesize an important metabolite and designing killswitches. In order to contain our bug, we have designed a bioreactor which will have several in built safety mechanisms. Some of the methods of containment include creating a closed system for the bioreactor which minimizes the escape of bacteria. Additionally, we will also be treating the belt-skimmer with ultra-violet light which will ensure that there are no bacteria in the final product.
In the rare instance that the bacteria escapes, we have designed a killswitch such that the bacteria is only able to survive in specific environments allowing them to perform the tasks of decarboxylation, denitrification and desulfurization in our bioreactor. However, in case of these bacteria escaping, the lack of a metabolite and or the presence of a particular metabolite will activate the “kill genes” which will cause the bacteria to self destruct. The killswitch mechanism was put in our system as a safety measure in addition to the bioreactor to contain the synthetic bacteria.
History:
Scientists have been trying to develop methods to limit bacterial viability and growth outside of the lab environment. One of the most popular methods used to ensure the safety of bacteria used in the lab was the creation of lab strain bacteria such as DH5α and Top10. These bacteria are metabolically deficient and are unable to survive outside of the lab environment without very specific nutrients. Additionally, The Registry of Biological Parts also has several killswitches readily available that were submitted by previous iGEM teams.
The different types of killswitches include:
Toxin-antitoxin systems:
These systems usually insert antitoxin in the plasmid and toxin in the genome. Ideally if the bacteria lost the plasmid then the bacteria dies. The advantages of these types of system is that__________ and the caveat with these systems is that they do not prevent the bacteria from horizontally transferring the genetic material.
Auxotrophic marker
Auxotrophes are bugs that are unable to survive in the absence of a metabolite. These bugs are used widely in the lab. An auxotrophe is unable to synthesize an essential metabolite, often an amino acid. Therefore, it requires the presence of the said metabolite in order to survive. Often these amino acids are unavailable in the environment. Therefore, these bugs are unable to survive outside the laboratory environment.
Inducible systems
Inducible systems generally consist of a regulatory element such as a promoter which is activated in the presence or absence of a metabolite. There is several kill genes inducible kill genes in the registry. Some of them include BamHI under the control of AraC promoter. The literature uses LacI promoter and the LacUV promoter as control elements. Some of the limitations of using an inducible system are the escaper bacteria mutating out either the kill gene or the regulatory element associated with the kill gene such as the promoter thereby blocking the expression of the kill gene. In order to combat this, researchers often create plasmids with multiple copies of the kill systems. This reduces the chances of mutation and also provides backup copies in case one of the promoters is mutated. Knudsen and Karlstorm suggest the use of a tightly controlled promoter to reduce the chances of mutation.
Design considerations:
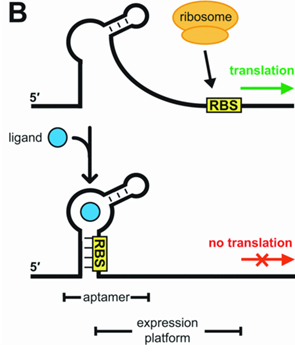
In our design we had considered all three of the possibilities however considering the large increase in cost in the bioreactor if auxotrophic systems were used, we decided to explore different inducible systems. We considered using the AraC promoter (Bba_I0500) as well as the LacI promoter (Bba_R0010) with our kill genes. However, data suggests that both AraC as well as LacI promoters are both leaky. PUT DIAGRAM. Therefore, we explored four inducible systems which are new to the registry and are induced by inexpensive ligands such as magnesium, manganese, molybate salts and glucose. In order to make sure the systems are controlled well and the kill switch regulation is not leaky, we have added an additional control using the riboswitch.
A riboswitch provides post-transcriptional control of gene expression. A riboswitch is a small stretch of mRNA which binds to a ligand which increases or decreases the expression of the gene downstream.
Our Kill Genes
The principal mechanism behind our active killswitch system are exo and endonucleases which work in tandem to cause substantial degradation of the bacterial genome. The chance event of bacterial escape from the bioreactor into tailings ponds triggers the transcription of S7 micrococcal nuclease and CviAII endonuclease.
Nuclease assay to evaluate the nucleases present in the registry (BglII and BamHI):
To compare S7 and CviAII to the nucleases already present in the registry we did a nuclease assay with commercially available enzymes from New England Biolabs. To see detailed protocol please see here
Regulation of our kill genes:
We have explored four different systems in our project. All of these systems fall under the umbrella of inducible kill gene systems. They are: Glucose repressible system, magnesium repressible system , manganese inducible system and the molybdate repressible system.
Manganese regulation
Similar to the magnesium system, the manganese system engages both a promoter and a riboswitch. However this system operates in a reciprocal manner compared to the magnesium system. Therefore when manganese is present in the system will allow expression of the system downstream and activate our kill genes S7 and CViAII. Additionally the manganese circuit also contains a transcriptional regulator (MntR). This is regulator containing a metal-binding domain that will in the presence of manganese repress the manganese ion transporter MntH preventing the bacteria from gaining the needed metals for survival.
Insert Figure. In this system when manganese is presence in the tailing ponds this will trigger the MntR regulator. As stated above this will prevent the movement of manganese into the bacteria as the MntH transporter will be repressed. In tandem the manganese riboswitch system with the MntA promoter and MntA riboswitch will be activated by the manganese allowing the kill gene downstream of it to be activated. Therefore use of both the MntR and the MntA riboswitch is to ensure double regulation.
Both the magnesium and manganese systems are both workable killswitch constructs however after analyzing the composition of the tailing ponds the systems will not be viable without a way to regulate these two metals in the contaminated waters.
Test circuits for the manganese system
Similar to the magnesium system the manganese will use a GFP LVA tag. The following are the control circuits built in order to characterise the MntA promoter and the MntA riboswitch.
Insert Circuits
TETR Promoter-RBS-GFP LVA
TETR Promoter-MntA Riboswitch- GFP LVA
MntA Promoter-RBS- GFP LVA
MntA Promoter-MntA Riboswitch-GFP LVA
MntA Promoter-MntA Riboswtich
Molybdate co-factor protein regulation
 "
"