Team:Groningen/Safety
From 2012.igem.org








Contents |
Safety in the lab
Working with GMO’s and microbiology is obligated to be done under safe conditions. The European union made a directive that insures the safety for the researcher, the laboratory and the environment. The directive however is very comprehensive and very detailed. To make a good and safe work environment the Rijksuniverisiteit Groningen(RUG) made an ’Administratieve organisatie GGOs’, this is based on the EU directive ‘98/81/EG’ and was approved by the ministry of Infrastructure & Environment from the Dutch government. The government is in turn advised by the COGEM, the scientific advise organ of the government. The COGEM provides scientific advice to the government on risks to human health and the environment of the production and use of GMOs. It also informs the government of ethical and societal issues linked to genetic modification.
Students studying microbiology at the university of Groningen are informed on safety aspects of microbiology from the beginning of their study career. They are required to get a VMT (Veilige microbriele technieken, translated: safe microbiological techniques) certificate that demonstrates that they are able to safely work with microbiology and GMOs. The bases for this certificate are the 10 commandments for safe microbiological techniques which are derived from the Administratieve organisatie GGOs. Every iGEM member from our team is required to know these rules and uphold them when working in the lab. These commandments are 10 rules which ensure safe work environment for researchers on a daily basis. Most of the team members that work in the lab have a background in microbiology which they learned at the RUG. These persons have the VMT certificate and know how to work safely in the lab. Team members without this background and who work in the lab have been instructed with these rules and learned how to work safely.
10 COMMANDMENTS for Safe Microbiological Techniques
- 1. All VMT related work can only be performed by those people that have permission from the Biological Safety Officer (BVF). Work according to the rules, even if you believe there is no apparent risk.
- 2. During VMT related work all doors and windows have to be closed. Verify that insects and other pests are not present in the lab.
- 3. Wear a closed laboratory coat. Do not take this labcoat outside the VMT area unless it is absolutely necessary for the experiment. In case of a contamination of the labcoat, sterilize the labcoat first, with bleach or by autoclaving, before washing.
- 4. Clean and sterilize spills immediately. Report serious contamination immediately to the BVF.
- 5. It is absolutely prohibited to eat, drink or smoke, or to have cups, plates, mugs or silverware in the VMT area.
- 6. Pipetting by mouth is prohibited. Used pipettes are collected in a disinfecting solution.
- 7. Prevent aerosols. These may be created by -splashing drops; -pouring of liquids; - discharging pipettes; -opening of moist plugs; - using inoculating loops that are too hot. Use needles only if there is absolutely no alternative.
- 8. Glassware and instruments that have been in contact with GMO's (Genetically Modified Organisms, GGO’s in Dutch) have to be sterilized or disinfected before being washed, reused or discarded. Biological waste has to be collected in autoclavable plastic bags, which are autoclaved before discarding (use indicator tape to demonstrate that the bag was autoclaved).
- 9. Wash hands with soap and water after work and before leaving the room. Bench surface areas have to be cleaned and disinfected daily. Keep area clean and organized.
- 10. Record the general nature of the work clearly in a lab journal.
More to consider
- Ethidium bromide is a carcinogenic compound that we used when working with agarose gels. To ensure our safety, we created a specific ethidium bromide region in our lab; in this area was clearly indicated that it is obligatory to use appropriate gloves and keep all the contaminated equipment in the EthBr area. The toxic wasted is disposed in the appropriated manner according the rule and regulations uphold in the laboratories of the university.
Micro-organisms involved
- We worked with bad meat, a versatile environment for the growth of Pseudomonas, Salmonella, E. coli and other harmful bacteria. In order to work as safely as possible, we performed our rotten meat experiments in closed bottles and used our flow cabinets and procedure masks when taking meat samples. All of the meat was assumed to be biological waste, so we put everything in the autoclave to ensure that our researchers worked as safe as possible. You can read more about food safety in the dedicated chapter below.
- Bacillus subtilis - For proof of principle we used B. subtilis strain 168, as safe strain widely used in labs. For our final product we want to use a food grade B. subtilis strain as Bacillus subtilis natto. Considered as safe as Lactococcus lactis in yoghurt.
- Escherichia coli strain DH5α- Standard laboratory bacterium, widely used as chassis for synthetic biology. This bacterium is considered moderately safe, however, may cause infections and therefore has to be treated carefully.
Genetical engineering and safety
Public safety
During our project discussions and our presentations to the public, it became clear that the association of meat and bacteria being close to each other, is not easily accepted. That’s why decided to take extra care when we designed our indication sticker, to be absolutely sure there is no possibility that our Bacillus or its spores are able to come in the environment or on the preserved meat, possibly endangering the public and environment. We consulted specialized companies to participate and contribute in our project using their knowledge of materials. A good idea for preventing introduction of GMO’s into the environment, is the use of nano pores. The material for this “sticker” is TPX® Polymethylpentene, a strong polymer that is will ensure no bacteria will not come in contact with the meat, but still allows the volatiles to reach the bacteria. This material is approved by FDA-standards and EU-food-standards.
But not only the sticker design ensures the safety of the public, also the nutrient composition in the sticker ensures Bacillus will only germinate and grow when the sticker is still intact. And even when our Bacillus is exposed to an environment that is favorable for growth, the production of the pigment will ensure that the bacterium kills itself, because the pigment is toxic for Bacillus. Further the strain we will use in our final product is considered a food-grade strain. The genetic modification done to this strain only consist of adding of a plasmid containing promoter regions from the genome of B. subtilis and parts from other biobricks that were found to have no dangerous effects for humans.
The find out more on the effect of our product on the public we want to contact the voedsel en waren authoriteit of the Dutch government.
Environmental safety
Our final product does not contain an antibiotic resistance marker. This is a main concern for environmental safety. Even in a worst case scenario, if our food warden bacterium is released into the environment, the plasmid will not provide an advantage for survival above the natural biodiversity. Nor could any genes potentially transferred to other bacteria give those bacteria the ability to become virulent or dominant over other bacteria, which in turn could endanger the biodiversity. The ability to produce a pigment will waste valuable energy of the bacteria and could in some cases be toxic for the cell. The strain we will use for our final product will also not threaten the biodiversity in the environment, because this strain a natural strain and already in the environment. For more insights of the safety aspects of our product we invited prof. Elsas, a member of COGEM and expert on the environmental aspect of biosafety. He will give us a lecture on the 6th of September
The BioBricks
We did not found urgent safety issues with our biobricks. The promoters were taken from the genome of B.subtilis 168 and are natural gene parts. Furthermore the other parts of our biobricks are parts taken from the biobricks in the registry. Also final product does not contain an antibiotic resistance marker.
The biosafety group
Every research facility has staff responsible for the safety of the labs where people work with GMO. The Rijksuniveriteit of Groningen is no exception. There are four biological safety officers each charged with a different aspects of safety (Site manager, Microorganisms, ecology and plants, animal safety) We invited Dr. Juke Lolkema, the safety officer in charge the safety in working with microorganisms, to give a lecture about GMOs and safety in the lab. Dr. Lolkema is responsible for the maintenance of safe microbial techniques in the whole life science building (Linneausborg). During this lecture we asked about the safety aspects of our project. He raised an issue we needed to address: how to kill the bacteria after use? The pigment product was observed to be toxic for certain bacteria we need to test whether is this the case for B. subtilis nato. If so the pigment will serve as a kill switch and kills the bacteria after use. If the pigment does not have a toxic effect on this strain of B. subtilis, we want to incorporate a kill switch in the plasmid that is activated after pigment production. In either case after pigment production the bacteria will be killed.
Food safety
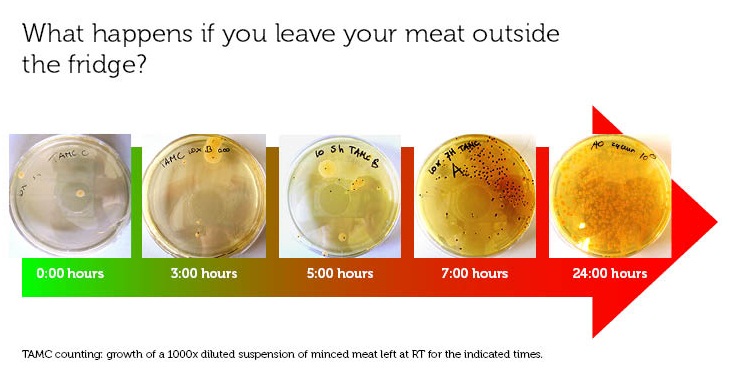
For our project, we did some research on food safety and the European regulations on this subject, especially around our subject, minced meat (results of research will follow). We took some of this regulations into practice by measuring the Total Aerobic Microbial Count of meat left at room temperature for different times. You can see the results here.
Safety issues and the future of the iGEM competition
Find an alternative system for the antibiotic resistance markers used nowadays (in the BioBricks). This could be a pigment instead of cloning bacteria with antibiotic resistance. By horizontal gene transfer these resistance genes can be passed to other bacterial species.
Use this page to answer the questions on the safety page.
 "
"