Team:TU Darmstadt/Labjournal/Degradation
From 2012.igem.org
(→Friday, 09.05.12) |
(→Friday, 09.05.12) |
||
| Line 425: | Line 425: | ||
==== Friday, 09.05.12 ==== | ==== Friday, 09.05.12 ==== | ||
* qualitative [https://2012.igem.org/Team:TU_Darmstadt/Protocols/Agarose_gel_electrophoresis Agarose gel electrophoresis] | * qualitative [https://2012.igem.org/Team:TU_Darmstadt/Protocols/Agarose_gel_electrophoresis Agarose gel electrophoresis] | ||
| - | |||
=== Week 4 / CW 20 === | === Week 4 / CW 20 === | ||
Revision as of 14:20, 24 September 2012
Contents
|
Degradation
This page features the work carried out by the degradation team. The main objectives were the production of a BioBrick containing Fusarium solani cutinase or Est13 esterase two enzymes potentially enabling E.coli of PET degradation and over-expression stems for activity screening.
Activity tests of [http://partsregistry.org/wiki/index.php?title=Part:BBa_K808032 BBa_K808032]
Week 1 / CW 35
Friday, 31.08.12
- Activity assay in DYT with DH5α containing [http://partsregistry.org/wiki/index.php?title=Part:BBa_K808032 BBa_K808032] (arabinose inducable RBS-PhoA-His6tag-pNBEst13-Myctag-EstA)
| Component | test tube 1 | test tube 2 | test tube 3 | test tube 4 |
|---|---|---|---|---|
| DYT-medium | 8 mL | 8 mL | 8 mL | 8 mL |
| PET particle | yes | yes | yes | yes |
| bacteria | yes | yes | yes | no |
| induced | 1.5% L-arabinose | 1.5% L-arabinose | no | no |
- test seemed to have worked: but an induced test tube without PET-granula was missing
Week 2 / CW 36
Tuesday, 04.09.12
- Activity assay in DYT with DH5α containing [http://partsregistry.org/wiki/index.php?title=Part:BBa_K808032 BBa_K808032]
| Component | test tube 1 | test tube 2 | test tube 3 | test tube 4 | test tube 5 |
|---|---|---|---|---|---|
| DYT-medium | 8 mL | 8 mL | 8 mL | 8 mL | 8 mL |
| PET particle | yes | yes | no | yes | yes |
| bacteria | yes | yes | yes | no | yes |
| induced | 1.5% L-arabinose | 1.5% L-arabinose | 1.5% L-arabinose | no | no |
- looks good but test tube 2 shows no significant change of colour
Wednesday, 05.09.12
- Activity assay in DYT with DH5α containing [http://partsregistry.org/wiki/index.php?title=Part:BBa_K808032 BBa_K808032]
| Component | tube 1 | tube 2 | tube 3 | tube 4 | tube 5 | tube 6 | tube 7 | tube 8 | tube 9 |
|---|---|---|---|---|---|---|---|---|---|
| DYT-medium | 8 mL | 8 mL | 8 mL | 8 mL | 8 mL | 8 mL | 8 mL | 8 mL | 8 mL |
| PET particle | yes | yes | yes | yes | no | no | yes | yes | yes |
| bacteria | yes | yes | yes | yes | yes | yes | yes | yes | no |
| induced | 1.5% L-arabinose | 1.5% L-arabinose | 1.5% L-arabinose | 1.5% L-arabinose | 1.5% L-arabinose | 1.5% L-arabinose | no | no | no |
- all induced tubes turned yellow, even without PET-granula as a substrate
- no more avtivity tests, we are awaiting the evaluation of test expression series of the [http://partsregistry.org/wiki/index.php?title=Part:BBa_K808000 arabinose inducible promoter] conjugated to GFP
Trouble shooting
- evaluation shows a very high sensisty of [http://partsregistry.org/wiki/index.php?title=Part:BBa_K808000 arabinose inducible promoter [BBa_K808000]] even to low concentrations of L-arabinose (expression starts at around 0.01%)
- induction of the DH5α with 1.5% arabinose ended fatal due to extrem expression of the transmembrane construct [http://partsregistry.org/wiki/index.php?title=Part:BBa_K808030 RBS-PhoA-His6tag-pNBEst13-Myctag-EstA]
- starting test expressions with lower L-arabinose concentrations ranging from 0.05% - 1%
Team II Kill Curves
Thursday, 06.09.12
- Activity assay in DYT with DH5α containing [http://partsregistry.org/wiki/index.php?title=Part:BBa_K808032 [BBa_K808032]] (arabinose inducable RBS-PhoA-His6tag-pNBEst13-Myctag-EstA)
| Component | tube 1 | tube 2 | tube 3 | tube 4 | tube 5 | tube 6 | tube 7 | tube 8 | tube 9 | tube 10 | tube 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DYT-medium | 5 mL | 5 mL | 5 mL | 5 mL | 5 mL | 5 mL | 5 mL | 5 mL | 5 mL | 5 mL | 5 mL |
| PET particle | yes | yes | yes | yes | yes | yes | no | no | no | no | no |
| bacteria | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | no |
| induced | 1% L-arabinose | 1% L-arabinose | 0.75% L-arabinose | 0.75% L-arabinose | no | no | 1% L-arabinose | 1% L-arabinose | 0.75% L-arabinose | 0.75% L-arabinose | no |
- all induced tubes turned yellow, even without PET-granula as a substrate
Week 3 / CW 37
Monday, 10.09.12
- Activity assay in DYT with DH5α containing [http://partsregistry.org/wiki/index.php?title=Part:BBa_K808032 [BBa_K808032]] (arabinose inducable RBS-PhoA-His6tag-pNBEst13-Myctag-EstA)
| Component | tube 1 | tube 2 | tube 3 | tube 4 | tube 5 | tube 6 | tube 7 | tube 8 | tube 9 | tube 10 | tube 11 | tube 12 | tube 13 | tube 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DYT-medium | 5 mL | 5 mL | 5 mL | 5 mL | 5 mL | 5 mL | 5 mL | 5 mL | 5 mL | 5 mL | 5 mL | 5 mL | 5 mL | 5 mL |
| PET particle | no | no | no | no | yes | yes | yes | yes | no | no | no | no | yes | yes |
| PET stripe | yes | yes | yes | yes | no | no | no | no | no | no | no | no | no | no |
| bacteria | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | no |
| induced | 0.05% L-arabinose | 0.1% L-arabinose | 0.2% L-arabinose | 0.4% L-arabinose | 0.05% L-arabinose | 0.1% L-arabinose | 0.2% L-arabinose | 0.4% L-arabinose | 0.05% L-arabinose | 0.1% L-arabinose | 0.2% L-arabinose | 0.4% L-arabinose | no | no |
- test tube 14: DYT without bacteria contains CAM, Kan, AMP
- 0.05% induced tubes with substrate show difference to tube without substrate
- quantification is possible via meassurement of absorption
- activity assay on LB-Tributyrin-CAM-plates with L-arabinose concentrations: 0.1%, 0.2%, 0.4%
Protein Expression
CW 24
Thursday, 14.06.2012
- PCR for protein expression of FsC
- each PCR is performed in 5 batches à 50 µL.
- Parameter: TA = 57°C, tA = 35 s, tE = 65 s
- PCR on pEST100 vector for expression with pEX vector
- gene of interest: FsC for designed part [http://partsregistry.org/wiki/index.php?title=Part:BBa_K808032 BBa_K808032]
- primers: pEX FsC His SfiI up and pEX FsC Stop SfiI lo
Friday, 15.06.2012
- Agarose gel electrophoresis for quality control

CW 25
Wednesday, 20.06.2012
- Agarose gel electrophoresis for preparation

- Gel extraction of PCR products
- Concentration of produced FsC sequence: 40 ng/µL
Thursday, 21.06.2012
- Restriction digest of PCR product from 14.06.
- Enzymes used: SfiI
- NEBuffer: 4
- Digestion time: over night
- Digestion temperature: 50°C
Friday, 22.06.2012
- Miniprep of pEX vector
- Concentration range: 240-488 ng/µL
- Restriction digest of pEX
- Enzymes used: SfiI
- NEBuffer: 4
- Digestion time: 3 days
- Digestion temperature: 50°C
CW 26
Monday, 25.06.2012 =
- Agarose gel electrophoresis for quality control

- Gel extraction of pEX
 and digested FsC sequence from 21.06.
and digested FsC sequence from 21.06. 
- Ligation of pEX with FsC sequence with the ratio 1:3 and 1:5
| Component | 1:3 | 1:5 |
|---|---|---|
| FsC sequence | 1.12 µL | 2.2 µL |
| pEX | 0.64 µL | 0.64 µL |
| Ligase buffer | 4 µL | 4 µL |
| T4 DNA ligase | 1 µL | 1 µL |
| H2O | 33 µL | 30 µL |
- Ligation time: over night
Tuesday, 26.06.2012
- Bacterial transformation by Electroporation of TOP10 with the ligation product pEX-FsC from 25.06.
Wednesday, 27.06.2012
Thursday, 28.06.2012
- Miniprep of pEX-Fsc in TOP10
- Concentration: 45 ng/µL and 405 ng/µL
- Bacterial transformation by Electroporation of BmH7118 with the Miniprep product pEX-FsC
CW 27
Monday, 02.07.2012
- Protein expression of FsC at different temperatures (16°C, 25°C, 30°C and 37°C) for the final step of the expression
Tuesday, 03.07.2012
- Purification of Periplasmatic Proteins for all expression temperatures
Wednesday, 04.07.2012
- Protein purification without cell disrupter
- SDS-PAGE (Schägger) analysis of all collected samples
| Expression at 16°C | Expression at 25°C |
|---|---|
 | 
|
| Expression at 30°C | Expression at 37°C |
 | 
|
- The best results were maintained at an expression temperature of 30°C
Friday, 06.07.2012
CW 28
Monday, 09.07.2012
- Miniprep of pEX-FsC from 06.07.
- Concentration: 470 ng/µL
- Preparation for sequencing at Eurofins
- Barcode 043 pEX-FsC with primer M13 Reverse up
- Barcode 044 pEX-FsC with primer ClaI pIII lo
- Plasmid DNA: 5µL
- Primer: 3µL
- H2O: 7µL
Wednesday, 11.07.2012
- Protein expression of FsC according to standard protocol
Thursday, 12.07.2012
- Protein purification by standard protocol
- SDS-PAGE (Schägger) analysis of samples

CW 29
Monday, 16.07.2012
- Protein expression of FsC according to standard protocol
Tuesday, 17.07.2012
- Protein purification by standard protocol
- SDS-PAGE (Schägger) analysis of samples

CW 31
Monday, 30.07.2012
- Protein expression of FsC according to standard protocol
Tuesday, 31.07.2012
- Protein purification by standard protocol
- SDS-PAGE (Schägger) analysis of samples

SOE PCR
Week 1 / CW 17
Tuesday, 24.04.12
- Production of electrocompetent cells DH5alpha and BL21
- Pouring of LB-Agar plates with ampecilin resistance (AMP)
- setting of DYT media
- Electroporation of BL21 with the following plasmids
Wednesday, 25.04.12
- Miniprep of the 3 overnigth Bl21 cultures and concentration meassurement via Nanodrop
Thursday, 26.04.12
Annotation: Every PCR is done in 4 assays à 50 µL, TA = 60°C, ta = 35s, tE = 25 s
- PCR on pEST100
- gene of interest: Promo-LacO-RBS-Phoa for SKV
- primers: SKV a1 up XbaI & SKV a1 lo NdeI
- PCR on pEST100
- gene of interest: Promo-LacO-RBS-Phoa for SOE PCR with pNB-Est13
- primers: SOE A up & SOE a1 lo
- PCR on pEST100
- gene of interest: Promo-LacO-RBS-Phoa for SOE PCR with FsC
- primers: SOE A up & SOE a2 lo
- PCR on pEST100
- gene of interest: FsC for SOE PCR with Promo-LacO-RBS-Phoa and EstA part1
- primers: SOE b2 up & SOE b2 lo
- PCR on pET26b(+)
- gene of interest: pNB-Est13 part1 for SOE PCR with Promo-LacO-RBS-Phoa and pNB-Est13 part2
- primers: SOE b1 up & SOE Est13 mut lo
- PCR on pET26b(+)
- gene of interest: pNB-Est13 part2 for SOE PCR with pNB-Est13 part1 and EstA part1
- primers: SOE Est13 mut up & SOE b1 lo
- Agarose gel electrophoresis (Agarose gele elektrophoresis) for qualitiy control
- 2-5 1.PCR, 6-9 2.PCR, 10-13 3.PCR
- 2-5 4.PCR, 6-9 5.PCR, 710-13. 6.PCR
Friday, 27.04.12
- clean up of 1. PCR on pEST100 from Thursday, 26.04.12 via Ammonium acetate - Ehtanol DNA precipitation, solved in 54 µl ddH2O
- restriction of cleaned up 1. PCR and pET16b with XbaI and NdeI over weekend at 37°C
Week 2 / CW 18
Monday, 30.04.12
- SOE PCR
- pNB-Est13 part1 & pNB-Est13 part2, primers: SOE b1 up & SOE b1 lo
- Promo-LacO-RBS-Phoa & FsC, primers: SOE A up & SOE b2 lo
- Annotation: Every PCR is done in 4 assays à 50 µL, TA = 60°C, ta = 35s, tE1 = 20s, tE1 = 35s
- Agarose gel electrophoresis of SOE PCR
- SOE PCR of Promo-LacO-RBS-Phoa-FsC worked, pNB-Est13 did not due to missing clean up via Agarose gel electrophoresis, precipitation is insufficient, we do it again
- PCRs
- PCR on pET26b(+)
- gene of interest: pNB-Est13 part1 for SOE PCR with Promo-LacO-RBS-Phoa and pNB-Est13 part2
- primers: SOE b1 up & SOE Est13 mut lo
- PCR on pET26b(+)
- gene of interest: pNB-Est13 part2 for SOE PCR with pNB-Est13 part1 and EstA part1
- primers: SOE Est13 mut up & SOE b1 lo
- Agarose gel electrophoresis
- 1. PCR worked well 2. PCR did not
Wednesday, 02.05.12
- PCRs
- PCR on pEST100
- gene of interest: Promo-LacO-RBS-Phoa for SOE PCR with pNB-Est13
- primers: SOE A up & SOE a1 lo
- PCR on pET26b(+)
- gene of interest: pNB-Est13 part1 for SOE PCR with Promo-LacO-RBS-Phoa and pNB-Est13 part2
- primers: SOE b1 up & SOE Est13 mut lo
- PCR on pET26b(+)
- gene of interest: pNB-Est13 part2 for SOE PCR with pNB-Est13 part1 and EstA part1
- primers: SOE Est13 mut up & SOE b1 lo
- TA = 57°C, ta = 35s, tE = 25 s
- every PCR is performed in 3 batches à 50 µL
- Promega gel extraction
- c(1.PCR)=6 ng/µL
- c(2.PCR)=12 ng/µL
- c(3.PCR)=13 ng/µL
- SOE PCR of 2.PCR and 3.PCR in order to form pNB-Est13 for SOE PCR with Promo-LacO-RBS-Phoa and EstA
- TA1 = 60°C, ta1 = 25 s, tE1 = 25 s
- TA = 60°C, ta2 = 25 s, tE2 = 35 s
Tuesday, 03.05.12
- preparative Agarose gel electrophoresis of SOE PCR from yesterday
- Gel extraction
- cpNB-Est13 7 ng/µL
Friday, 04.05.12
- PCRs
- PCR on pEST100
- gene of interest: Promo-LacO-RBS-Phoa for SOE PCR with pNB-Est13
- primers: SOE A up & SOE a1 lo
- PCR on pEST100
- gene of interest: EstA part1 for SOE PCR with Promo-LacO-RBS-Phoa and pNB-Est13
- primers: SOE c1 up & SOE EstA mut PstI out lo
- PCR on pEST100
- gene of interest: EstA part1 for SOE PCR with Promo-LacO-RBS-Phoa and FsC
- primers: SOE c2 up & SOE EstA mut PstI out lo
- PCR on pEST100
- gene of interest: EstA part2 for SOE PCR with EstA part1
- primers: SOE EstA mut PstI out up & SOE D lo
- annotation: TA = 60°C, ta = 25 s, tE = 35 s
- every PCR is performed in 3 batches à 50 µL
- preparative Agarose gel electrophoresis
Week 3 / CW 19
Tuesday 08.05.12
- gene of interest: Promo-LacO-RBS-Phoa-pNBEst13 for SOE PCR with EstA
- primers: SOE A up & SOE b1 lo
- template: pNB-Est13 from last friday & Promo-LacO-RBS-Phoa from Wednesday, 02.05.12
- annotation: TA1 = 60°C, ta1 = 25 s, tE1 = 35 s
- TA2 = 60°C, ta2 = 25 s, tE2 = 1 min
- did not work
Wednesday, 09.05.12
- PCR on pET26b(+)
- gene of interest: pNB-Est13 part1 for SOE PCR with Promo-LacO-RBS-Phoa and pNB-Est13 part2
- primers: SOE b1 up & SOE Est13 mut lo
- PCR on pET26b(+)
- gene of interest: pNB-Est13 part2 for SOE PCR with pNB-Est13 part1 and EstA part1
- primers: SOE Est13 mut up & SOE b1 lo
- PCR on pEST100
- gene of interest: EstA part1 for SOE PCR with Promo-LacO-RBS-Phoa and pNB-Est13
- primers: SOE c1 up & SOE EstA mut PstI out lo
- PCR on pEST100
- gene of interest: EstA part1 for SOE PCR with Promo-LacO-RBS-Phoa and FsC
- primers: SOE c2 up & SOE EstA mut PstI out lo
- PCR on pEST100
- gene of interest: EstA part2 for SOE PCR with EstA part1
- primers: SOE EstA mut PstI out up & SOE D lo
- annotation: TA = 60°C, ta = 25 s, tE = 35 s
- every PCR is performed in 2 batches à 50 µL
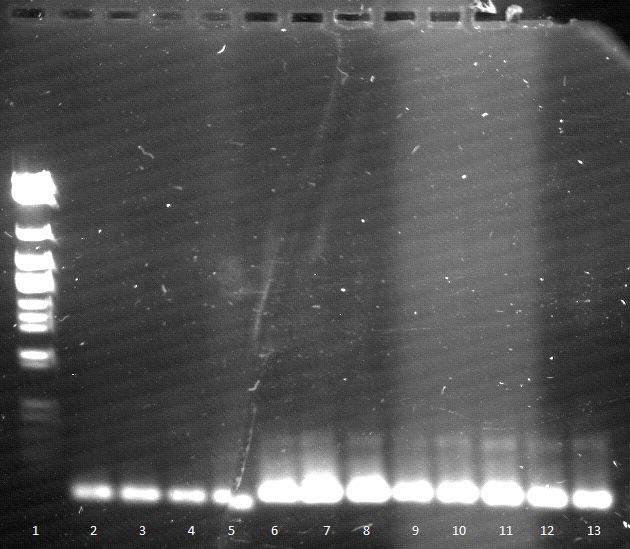
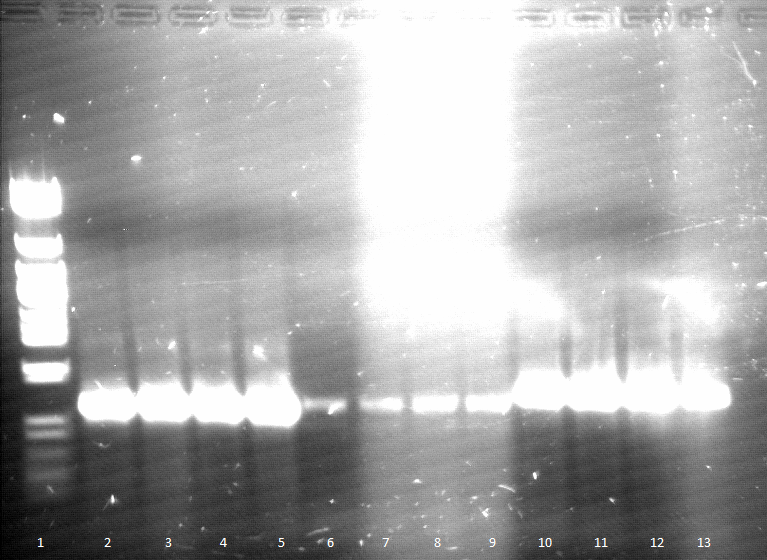
Friday, 09.05.12
- qualitative Agarose gel electrophoresis
Week 4 / CW 20
Monday, 14.05.12
- Gel extraction of remaining PCRs from Wednesday, 09.05.12
- c(pNB-Est13 part1)=24 ng/µL
- c(pNB-Est13 part2)=26 ng/µL
- c(EstA part2)=13 ng/µL
- new SOE EstA mut PstI out lo is orderd from Sigma-Aldrich
- SOE PCR
- gene of interest: pNB-Est13 for SOE PCR with EstA and Promo-LacO-RBS-Phoa
- primers: SOE b1 up & SOE b1 lo
- template: pNB-Est13 part1 from last friday & pNB-Est13 part2
- annotation: TA1 = 52°C, ta1 = 25 s, tE1 = 35 s
- TA2 = 57°C, ta2 = 25 s, tE2 = 1.15 min
- Agarose gel electrophoresis
Tuesday, 15.05.12
- SOE PCR
- gene of interest: Promo-LacO-RBS-Phoa-pNBEst13 for SOE PCR with EstA
- primers: SOE A up & SOE b1 lo
- template: pNB-Est13 from yesterday & Promo-LacO-RBS-Phoa from Wednesday, 02.05.12
- SOE PCR
- gene of interest: Promo-LacO-RBS-Phoa-FsC for SOE PCR with EstA
- primers: SOE A up & SOE b2 lo
- template: FsC from Thursday, 26.04.12 & Promo-LacO-RBS-Phoa from Wednesday, 02.05.12
- annotation: TA1 = 52°C, ta1 = 25 s, tE1 = 35 s
- TA2 = 57°C, ta2 = 25 s, tE2 = 1.15 min
- each PCR is performed in 2 batches à 50 µL
Wednesday, 16.05.12
- SOE PCR of [[Promo-LacO-RBS-Phoa-pNBEst13] did not work but Promo-LacO-RBS-Phoa-FsC worked
- Gel extraction
- c(Promo-LacO-RBS-Phoa-FsC)=10 ng/µL
Week 5 / Kw 21
Monday, 23.05.12
- PCR on pEST100
- gene of interest: EstA part1 for SOE PCR with pNB-Est13 and EstA part2
- primers: SOE c1 up & SOE EstA mut PstI out lo
- PCR on pEST100
- gene of interest: EstA part1 for SOE PCR with FsC and EstA part2
- primers: SOE c2 up & SOE EstA mut PstI out lo
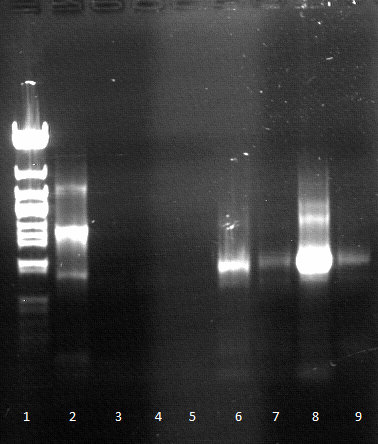
Tuesday, 22.05.12
- qualitative Agarose gel electrophoresis
- Gel extraction
- c(EstA part1 for SOE PCR with FsC)=4 ng/µL
- c(EstA part1 for SOE PCR with pNB-Est13)=7 ng/µL
Wednesday, 23.05.12
- SOE PCR
- gene of interest: EstA for SOE PCR with pNB-Est13
- primers: SOE c1 up & SOE D lo
- template: EstA part1 from yesterday & EstA part2 from Monday, 14.05.12
- SOE PCR
- gene of interest: EstA for SOE PCR with FsC
- primers: SOE c2 up & SOE D lo
- template: EstA part1 from yesterday & EstA part2 from Monday, 14.05.12
- annotation: TA1 = 52°C, ta1 = 25 s, tE1 =45 s
- TA2 = 65°C, ta2 = 25 s, tE2 = 90 s
- qualitative Agarose gel electrophoresis
Thursday, 24.05.12
- PCR on EstA for SOE PCR with pNB-Est13 from yesterday
- PCR on EstA for SOE PCR with FsC
- PCR on pEST100
- gene of interest: Promo-LacO-RBS-Phoa for SOE PCR with pNB-Est13
- primers: SOE A up & SOE a1 lo
- PCR on pEST100
- gene of interest: FsC for SOE PCR with Promo-LacO-RBS-Phoa and EstA part1
- primers: SOE b2 up & SOE b2 lo
- annotation: TA = 60°C, ta = 25 s, tE =45 s
- each PCR is performed in 3 batches à 50 µL
- qualitative Agarose gel electrophoresis
- both EstAs worked
- Promo-LacO-RBS-Phoa for SOE PCR with pNB-Est13 worked
- Gel extraction
Week 6 / Kw 22
Tuesday, 29.05.12
- SOE PCR
- gene of interest: Promo-LacO-RBS-Phoa-Fsc-EstA
- primers: SOE A up & SOE D lo
- template: EstA from Thursday, 24.05.12 & Promo-LacO-RBS-Phoa-Fsc from Wednesday, 16.05.12
- SOE PCR
- gene of interest: Promo-LacO-RBS-Phoa-pNBEst13-EstA
- primers: SOE A up & SOE D lo
- template: EstA from Thursday, 24.05.12 & Promo-LacO-RBS-Phoa from Wednesday, 02.05.12 & pNB-Est13 from Monday, 14.05.12
- SOE PCR
- gene of interest: Promo-LacO-RBS-Phoa-pNBEst13 for SOE PCR with EstA
- primers: SOE A up & SOE b1 lo
- template: Promo-LacO-RBS-Phoa from Wednesday, 02.05.12 & pNB-Est13 from Monday, 14.05.12
- annotation: each PCR is performed in 3 batches à 50 µL
- TA1 = 52°C, ta1 = 25 s, tE1 =45 s
- TA2 = 65°C, ta2 = 25 s, tE2 = 90 s
- Agarose gel electrophoresis
- Promo-LacO-RBS-Phoa-Fsc-EstA worked
- Promo-LacO-RBS-Phoa-pNBEst13 worked
Thursday, 29.05.12
- Gel extraction
- c(Promo-LacO-RBS-Phoa-Fsc-EstA)= 8 ng/µL
- c(Promo-LacO-RBS-Phoa-pNBEst13)= 13 ng/µL
SKV
Week 1 / CW 17
Tuesday, 24.04.12
- Production of electrocompetent cells DH5alpha and BL21
- Pouring of LB-Agar plates with ampecilin resistance (AMP)
- setting of DYT media
- Electroporation of BL21 with the following plasmids
Wednesday, 25.04.12
- Miniprep of the 3 overnigth Bl21 cultures and concentration meassurement via Nanodrop
Thursday, 26.04.12
Annotation: Every PCR is done in 4 assays à 50 µL, TA = 60°C, ta = 35s, tE = 25 s
- PCR on pEST100
- gene of interest: Promo-LacO-RBS-Phoa for SKV
- primers: SKV a1 up XbaI & SKV a1 lo NdeI
- Agarose gel electrophoresis (Agarose gele elektrophoresis) for qualitiy control
120426 PCR 1-3 siehe Laborbuch 26.4.tif
Friday, 27.04.12
- clean up of 1. PCR on pEST100 from Thursday, 26.04.12 via Ammonium acetate - Ehtanol DNA precipitation, solved in 54 µl ddH2O
- restriction of cleaned up 1. PCR and pET16b with XbaI and NdeI over weekend at 37°C
Week 2 / CW 18
Monday, 30.04.12
- Agarose gel electrophoresis of DNA digestion from Friday, 27.04.12
120430 Testrestrikt.XbaINdeI.tif
- Only one single band on Agarose gel electrophoresis image
- For saveness two single digests are performed at 20 µL batches. One with XbaI and the other with NdeI. Incubation for 2 hours.
- Agarose gel electrophoresis of single digests
- Enzymes cut once each, so digest should have worked.
- Ligation of PhoA in cut (XbaI / NdeI) pET16b(+)
- Annotation: 30 µL batch, 1:5 ratio, Ligation at 4°C for 2 days till Wednesday, 02.05.12
Week 4/CW 20
Monday, 07.05.
- Electroporation of DH5alpha with 5 µl of the ligation which was performed 2 days before.
- clean up of the PCR-product PhoA Friday, 04.05.
- Restriction digest of half of the product with XbaI/NdeI for 1,5h at 37°C
- PhoA is cleaned up by Agarose gel electrophoresis
- concentration:7,36 ng/µl,10,6 ng/µl
- Ligation of PhoA x pET16b(+) for 2h, at the rate of (Vector/Insert) 1:5, 1:3
Annotation: If it does not say anything else, Ligation is always done in 20µl batches.
Tuesday, 08.05.
- Transformation of DH5alpha with PhoA x pET16b(+) was not successfull, so it was performed again Monday, 07.05.
- DNA digestion of pET16b(+)
- 100 µl are cut with XbaI/NdeI, incubaton-time: 1,5h at 37°C
- product is cleaned up by Ammonium acetate - Ehtanol DNA precipitation
- concentration: 85 ng/µl
Wednesday, 09.05.
- Colony-PCR
- gene of interest: PhoA
- primer:SKV a1 up XbaI, SKV a1 lo NdeI
- PhoA was not inserted, but 2 colonies were picked and inoculated in 5 ml LB Amp medium to look at it again due to a test-restriction.
Thursday, 10.05.
- Miniprep of PhoA x pET16b(+)
- Restriction digest of the Miniprep: PhoA x pET16b(+)
- enzymes: XbaI/NdeI, incubation: 1,5 h, 37°C
- digest shows that PhoA was not inserted
- Ligation is performed again Monday, 07.05.
- Amplification of the genes FSC, Est13 and EstA via PCR gg 1
- templates:
- primer:
Friday, 11.05.
- DNA digestion of pET16b(+) to produce template for a new ligation of PhoA
- concentration: 76,3 ng/µl
- enzymes: XbaI, NdeI
- incubation: for 2 days, 37°C
- LIgation of PhoA x pET16b(+) cut with XbaI and NdeI is carried out using different rates of Vector and insert:
- ratio vector/insert= 3:1, 1:1, 1:3, 1:5, 1:7, 1:10
- PhoA= 10,6 ng/µl Monday,07.05.
- incubation: 2 days, 4°C
Week5/CW21
Monday, 14.05.
- the Restriction digest of pET16b(+) cut with XbaI and NdeI is isolated by Ammonium acetate - Ehtanol DNA precipitation
- LIgation of phoA x pET16b(+) is used to transform DH5alpha via Electroporation
Tuesday, 15.05.
- Colony-PCR
- primer:
- SKV a1 up XbaI,
- SKV a1 lo NdeI

- PhoA is inserted, so 10 clones are picked to grow them in 5ml DYT over night.
Wednesday, 16.05.
- Miniprep of 10 clones, the plasmid-DNA is isolated out of 3ml culture per clone.
- Restriction digest with NdeI and NcoI of the plasmids PhoA x pET16b(+) 1-4, so FSC and Est13 can be added to the construct.
- FSC is cut with NdeI and NcoI
- digests are performed in 20µl total volume at 37°C for 1,5h and cleaned up by Agarose gel electrophoresis
-

- PCR1 of EST13
- template: product of SOE-PCR, cleaned up by the Promega-Kit
- primer: SKV b1 up NdeI, SKV b1 lo NcoI
Friday, 18.05.
- Ligation of FSC x (phoA x pET16b(+))
- ratio vector/insert: 3:1, 1:1, 1:3
- concentration:
- (phoA x pET16b(+))= 3,72 ng/µl
- FSC= 12,22 ng/µl
Week 6/CW 22
Monday, 21.05.
- Elektroporation of DH5alpha with the Ligation of FSC x (phoA x pET16b(+))
- Restriction digest of (phoA x pET16b(+))(150µl,160µl) for the insertion of Est13/FSC and Est13(50µl)
- enzymes: NdeI, NcoI
- incubation: 1,5h, 37°C
- cleaned up viaAgarose gel electrophoresis
- c(phoA x pET16b(+))= 23,5 ng/µl
- c(Est13= 7,7 ng/µ
Tuesday, 22.05.
- Ligation of Est13 and (phoA x pET16b(+))
- both components have been cut with NdeI and NcoI Monday, 21.05.
- ratio vector/insert: 1:10, 1:5, 1:3
- incubation: 2h, 25°C
- Electroporation of dH5alpha using the Ligation of Est13 x (phoA x pET16b(+), 5 µl per 100µl cells
Wednesday, 23.05.
- colony-PCR
- aim is to check the insertion of Est13
- Primer:SKV b1 up NdeI, SKV b1 lo NcoI
- 8 colonies have been picked and the Agarose gel electrophoresis proves, that Est 13 has not been inserted into (phoA x pET16b(+)
Wednesday, 23.05.
- Ligation of FSC and Est13 in (phoA x pET16b(+)
- ratio vector/insert: 1:3, 1:5
- c(templates):Monday, 21.05.
- Elektroporation of DH5alpha with Est13 in (phoA x pET16b(+)
Thursday, 24.05.
- colony-PCR proves, that Est13 has been inserted into (phoA x pET16b(+)
- plates are covered with colonies, 8 colonies are chosen for colony-PCR
- colony No.5 is chosen to be inoculated in 5µ DYT/Amp medium
- [PCR 1] is performed again to amplify FSC and Est13
- products are cleaned up by Agarose gel electrophoresis

Friday, 25.05.
- Restriction digest of FSC and Est13 Thursday, 24.05.
- enzymes: NdeI, NcoI
- incubation: 2days, 37°C
week 7/CW 23
Tuesday, 29.05.
- Restriction digest of FSC and Est13 is cleaned up via Agarose gel electrophoresis
- MIniprep of Est13 x (phoA x pET16b(+) via Promega-Kit
- Ligation of FSC x (phoA x pET16b(+))
- ratio vector/insert: 1:3, 1:5, 1:10
- incubation: 2h, 25°C
- Electroporation of DH5alpha with 5µl of the LIgaton: FSC x (phoA x pET16b(+)
- PCR 1 to amplify EstA, (Thursday, 10.05.)
- 4 assays à 50 µL, TA = 66°C, ta = 35s, tE = 90s
- cleaned up via Kit
Wednesday, 30.05.
- Restriction digest of EstA (product of PCR, 80 µl) and Plasmid: (Est13 x phoA x pET16b(+))
- enzymes: EcoRI, NcoI
- incubation: 1,5h, 37°C
- Agarose gel electrophoresis
-

- c(EstA) = 6,7 ng/µl
- c(Est13 x phoA x pET16b(+))= 23 ng/µl
- Electroporation of FSC x (phoA x pET16b(+) worked well, plates are covered with colonies which are picked to be grown in 5ml DYT/Amp medium over night.
Thursday, 31.05.
- Miniprep of the amplified (FSC x phoA x pET16b(+))
- Restriction digest to ligate EstA
- enzymes:NcoI, EcoRI
- incubation: 1,5h, 37°C
- to be sure that the plasmid (Est13 x phoA x pET16b(+)) really carries Est13 and PhoA, an analytic PCR1 is performed. Also pEt16b(+) is used as template with different primers.
- templates:
- primers, used on each template:
- conditions:
- TA = 66°C, ta = 35s, tE = 90s
- the Agarose gel electrophoresis shows, that there is no binding-specifity concerning the genes of interest and the primers, but primers have been checked on mistakes.
- to solve the problem, the LIgation of FSC and Est13 shall be repeated from the beginning.
-

- Additionally,the decision was to go on with the Ligation of EstA into the Plasmids (Est13 x phoA x pET16b(+)) and (FSC x phoA x pET16b(+)), in case that the PCR was proceeded under wrong conditions.
Friday, 01.06.
- Restriction digest of (phoA x pET16b(+))
- enzymes: NcoI, NdeI
- incubation: 1,5 h, 37°C
- LIgation of Est13 and FSCx(PhoAxpET16b(+))
- Restriction digest of (FSCx (PhoA x pET16b(+))
- enzymes: NcoI, EcoRI
- incubation: 1,5h, 37°C
week 8/CW 24
Monday, 04.06.
- restriction of (FSCx(PhoAxpET16b(+)) and (phoAxpET16b(+)) is cleaned up by Agarose gel electrophoresis
- Electroporation of DH5alpha with Ligation: (FSCxPhoAxpET16b(+)), (EST13xPhoAxpET16b(+)) Friday, 01.06
Tuesday, 05.06.
- colony-PCR on EST13 & FSC is negative.
Wednesday, 06.06.
- Electroporation of DH5alpha with Ligation-assays from 01.06.
- Ligation of EstAWednesday,30.05 and the Plasmids (Est13xphoAxpET16b(+)),(FSCxphoAxpET16b(+)) from Monday,04.06.
Friday, 08.06.
- No cells are grown on plates, so the plates are incubated for another two days at RT.
week8/ CW24
Monday, 11.06.
- all plates are covered with cells, so a colony-PCR is performed on either Est13,FSC or EstA
- primer:
- SKV b1 up NdeI, SKV b1 lo NcoI
- SKV b2 up NdeI, SKV b2 lo NCOI
- SKV c1 up NcoI, SKV c1 lo EcoRI
- Every PCR is done in 8 assays à 50 µL, TA = 57°C, ta = 35s, tE = 90 s
Tuesday, 12.06.
- Agarose gel electrophoresis shows, that EstA has been inserted in both Plasmids, (Est13xPhoAxpET16b(+)) and (FSCxPhoAxpET16b(+))
-

- colonies No.3,4 (EstAxFSCxPhoAxpET16b(+)) and No.15,16 (EstAxEst13xPhoAxpET16b(+)) seem to be positive on EstA and are picked to be grown in 5ml DYT/Amp medium.
- colony-PCR on Est13 and FSC shows a lot of unspecific bands, so cloning of the genes from the beginning has to be dropped.
Wednesday, 13.06.
- Miniprep of the clones No. 3,4,15 and 16. Tuesday, 12.06.
- for gene-expression, it is performed a Elektroporation of BL21-cells with 1µl of the PLasmid-DNA which has just been isolated.
Friday, 15.06.
- Transformation of BL21 worked well
- Tributyrinagar plates with 1mM IPTG were generated, to induce the Lac-Promotor of pET16b(+), and to demonstrate the esterase-activity by the lysis of Tributyrin.
- afterwards Tributyrinagar plates are inoculated with cells which were transformed by Miniprep No.3,4,15,16 on Wednesday, 13.06.
- incubation: 37°C, 2 days
week9/CW25
Monday, 18.06.
- Tributyrinagar plates are covered with cells, but no lysis is visible.
- plates are incubated for another day at 25°C.
Tuesday, 19.06.
- no lysis, plates stay incubated at 25°C.
Wednesday, 20.06.
- no lysis
Friday, 22.06.
- The plasmids No.3,4 (EstAxFSCxPhoAxpET16b(+)) and No.15,16 (EstAxEst13xPhoAxpET16b(+)) are prepared for sequencing at Seqlab
- primer:
- (EstAxFSCxPhoAxpET16b(+)):
- (EstAxEst13xPhoAxpET16b(+)):
Sequencing was not successfull. It was discovered later, that there was a contamination of the DH5alpha cells with other plasmid-DNA, probably caused by Electroporation. Verlinkung Arne Due to this fact, the isolated plasmids were always mixed up with other plasmids, and sequencing had to fail. The contamination of the cells may have also caused false-positive results of the colony-PCRs.
We suggest that the cutinase FSC has an additional lipase-activity, which causes a lysis of the cell-membrane and may have an selective effect on FSC-negative mutants while cultivating the cells.
BioBricks
Week 1 / Kw 29
Tuesday, 17.07.12
- each PCR is performed in 5 batches à 50 µL.
- TA = 57°C, tA = 30 s, tE = 2 min
- PCR on pEST100
- gene of interest: PhoA for designing part BBa_K808028
- primers: BBa PhoA up & BBa PhoA down
- PCR on pET26b(+)
- gene of interest: pNB-Est13 part1 for designing part BBa_K808026 via SOE PCR
- primers: BBa Est13 up & SOE Est13 mut lo
- PCR on pET26b(+)
- gene of interest: pNB-Est13 part2 for designing part BBa_K808026 via SOE PCR
- primers: BBa Est13 down & SOE Est13 mut up
- Agarose gel electrophoresis for quality control
- preparative Agarose gel electrophoresis
- Gel extraction of PCR products
- 1. PCR (gene of interest: phoA): c(PhoA)=19 ng/µL
- 2. PCR (gene of interest: pNB-Est13 part1): c(pNB-Est13 part1)=39 ng/µL
- 3. PCR (gene of interest: pNB-Est13 part2): c(pNB-Est13 part2)=40 ng/µL
- SOE PCR
- gene of interest: pNB-Est13 for designing part BBa_K808028
- primers: BBa Est13 up & BBa Est13 down
- Annotation: SOE PCR is performed in 5 batches à 50 µL
- TA1 = 52°C, tA1 = 25 s, tE1 = 75 s
- TA2 = 57°C, tA2 = 30 s, tE2 = 2 min
- stored over night at 10°C
Wednesday, 18.07.12
- qualitative Agarose gel electrophoresis of SOE PCR (from Tuesday, 17.07.12)
- preperative Agarose gel electrophoresis
- Gel extraction of SOE PCR produkt
- PCR 1
- gene of interest: EstA from SKV Date for designing part BBa_K808027
- primers: BBa EstA up and BBa EstA down
- Annotation: PCR is performed in 5 batches à 50 µL
- TA = 57°C, tA = 25 s, tE = 75 s
- GC Buffer is used
- preparative Agarose gel electrophoresis
- Gel extraction of PCR product
- gene of interest: EstA: c(pNB-Est13 part2)=119 ng/µL
- stored in freezer
Thursday, 19.07.12
- inoculation of 5 mL DYT-media with 5 µL CAM and DH5alpha carrying pSB1C3 with part BBa_K808025 (FsC)
- DNA Digestion of the following parts
- PhoA from Tuesday, 17.07.12
- pNB-Est13 fromTuesday, 17.07.12
- EstA from Wednesday, 18.07.12
- pSB1C31
- pSB1C32
- adding of Dephosphatase Antarctica from NEB on digested pSB1C3 1 & 2
- Ligation into digested and 5' dephosphorylated pSB1C3 1
- bacterial transformation by Electroporation of DH5alpha
Friday, 20.07.12
- the following Electroporation from Thursday, 19.07.12 worked
- inoculation of 5 mL DYT-media with 5 µL CAM and two positive colonies from each transformation (from pNB-Est13 3 colonies are taken) .
- in addition FsC is already on plate, but to controle it, 2 colony PCRs are performed
- Ligation of EstA into digested and 5' dephosphorylated pSB1C3
- Electroporation of DH5alpha
- Miniprep of inoculated FsC in pSB1C3 (BBa_K808025) from Thursday, 19.07.12 failed
- inoculation of 5 mL DYT-media with 5 µL CAM and DH5alpha carrying pSB1C3 with part BBa_K808025 (FsC)
- incubation at 37°C over weekend of transformations and picked colonies
Week 2 / CW 30
Monday, 23.07.12
- Colony PCR of picked colonies from Friday, 20.07.12
120723 BBaPhoA BBa PhoA BBa FsC BBa FsC BBa Est13 BBa Est13 BBa Est13.jpg
- 2-3 PhoA colonies, 4-5 FsC colonies, 6-8 pNB-Est13 colonies
- Miniprep of one inoculated colony in DYT-media with CAM from Friday, 20.07.12
- inoculation of 2 x 5 mL DYT-media-CAM with pSB1C3 carrying FsC
- second transformation of EstA from Friday, 20.07.12 worked, but too many colonies!
- purity plate is done
Tuesday, 24.07.12
- Miniprep of the 2 FsC DYT-media-CAM cultures with pSB1C3 carrying FsC from yesterday
- purity plate of EstA in pSB1c3 is positive
- colonies picked
- Colony PCR with positive control by amplfifying an SKV-EstA
- Annotation: TA = 57°C, tA = 25 s, tE = 2 min, done with NEB- Phusion so its similiar to PCR 1
- GC Buffer is used
Wednesday, 25.07.12
- Agarose gel electrophoresis of Colony PCR
- worked out well
120725 Colony BBaEstA positiv probe auf SKV EstA.jpg
Thursday, 26.07.12
- Sequencing is ordered. The following premixes are used :
- 10 µL of c(PhoA in pSB1C3)=220 ng/µL, 1µL VR, 9 µL ddH2O
- 10 µL of c(PhoA in pSB1C3)=220 ng/µL, 1µL VF2, 9 µL ddH2O
- 7 µL of c(FsC in pSB1C3)=168 ng/µL, 1µL VR, 7 µL ddH2O
- 7 µL of c(FsC in pSB1C3)=168 ng/µL, 1µL VF2, 7 µL ddH2O
- 5 µL of c(EstA in pSB1C3)=350 ng/µL, 1µL VR, 9 µL ddH2O
- 5 µL of c(EstA in pSB1C3)=350 ng/µL, 1µL VF2, 9 µL ddH2O
- 5 µL of c(pNB-Est13 in pSB1C3)=50 ng/µL, 1µL VR, 9 µL ddH2O
- 5 µL of c(pNB-Est13 in pSB1C3)=50 ng/µL, 1µL VF2, 9 µL ddH2O
- in order to prepare DMSO stocks and Minipreps 5 mL DYT-media-CAM are inoculated with following colonies containing
Friday, 27.07.12
- inoculated pNB-Est13 in pSB1C3 from Thursday, 26.07.12 has not grown over night
- new colony pcked from plate and incubated in 5 mL DYT-media-CAM at 37°C over night
- Miniprep of 3 mL from the following over night DYT-media cultures from Thursday, 26.07.12
Week 3 / CW 31
Monday, 30.07.12
- bacterial transformation by Electroporation of DH5alpha with c(pNB-Est13 in pSB1C3)=50 ng/µL from Monday, 23.07.12
Tuesday, 31.07.12
- no results in sequencing our BioBricks / parts from Thursday, 26.07.12 except for FsC in pSB1C3
- new Colony PCR(similar to PCR 2) is done on
- no signal on qualitative Agarose gel electrophoresis
- Trouble shooting (see discussion below)
Trouble shooting
- Symptoms
- very much transformants on crossed out plates after bacterial transformation by Electroporation
- at first Colony PCR is positive
- Minipreps result in very high yields (exceeding >200 ng/µL)
- second Colony PCR is negative
- sequencing failed
- BUT FsC in pSB1C3
- has had not very much transformants
- has had a seuqencing result
- its Minipreps resulted in medium concentrations, settling around 90 ng/µL
- Diagnosis:
- our bacterial transformation is done by Electroporation
- therefor we use electroporation cuvettes, stored in DNA precipitating liquids like isopropanol or ethanol after being washed out.
- long before we started these electroporation cuvettes were in use.
- if not washed out carefully, DNA debris (like vectors and Inserts) accumulates in these storage liquids, due to precipitation
- when an Electroporation is performed with one of these cuvettes, probably containing only little DNA debris, the cells get transformed with numbers of different precipitated plasmids
- these plasmids contain a variety of resistances, reaching from CAM over KAN to Amp, allowing even "wrong" transformants to grow on selective media
- after a short period of time, bacterial colonies start to seperate and repel their plasmids, selecting only the most "comfortables"
- probably our plasmids, containing big genes on ligated vectors, are pushed out during this phase of selection
- this slow decrease of plasmid could explain the missing second positive signal of Colony PCR
- Strategy
- switching our method from Electroporation to Heatshock transformation
Wednesday, 01.08.12
- due to change of strategy the following PCR 1s are performed
- PCR on pEST100
- gene of interest: PhoA BioBrick for assembly of part BBa_K808028
- primers: BBa PhoA up & BBa PhoA down
- PCR on pEST100
- gene of interest: EstA part1 for SOE PCR of EstA (BBa_K808027)
- primers: BBa Est A up & SOE EstA mut PstI out lo
- PCR on pEST100
- gene of interest: EstA part2 for SOE PCR of EstA (BBa_K808027)
- primers: BBa EstA mut up & BBa EstA down
- PCR on pET26b(+)
- gene of interest: pNB-Est13 part1 for SOE PCR of pNB-Est13 (BBa_K808026)
- primers: BBa Est13 up & BBa Est13 mut lo
- PCR on pET26b(+)
- gene of interest: pNB-Est13 part2 for SOE PCR of pNB-Est13 (BBa_K808026)
- primers: BBa Est13 lo & BBa Est13 mut up
- Annotation: every PCR is performed in 3 batches à 50 µL
- TA = 57°C, tA = 25 s, tE = 75 s
- due to time pressure there will be no qualitative but only preperative Agarose gel electrophoresis
- PhoA only on analytical scale but on same Agarose gel electrophoresis with EstA part1
120801 soe pcr esta1 pcr phoa.jpg
- 1 cut out EstA part1, 2 PhoA
120801 soe pcr est131 est132.jpg
- 2 cut out pNB-Est13 part1, 3 cut out pNB-Est13 part2
- cut out EstA part2
- Gel extraction of EstA part1, EstA part2, pNB-Est13 part1 and pNB-Est13 part2
- c(EstA part1)=19 ng/µL
- c(EstA part2)=110 ng/µL
- c(pNB-Est13 part1)=45 ng/µL
- c(pNB-Est13 part2)=48 ng/µL
- SOE PCRs
- SOE PCR
- gene of interest: EstA
- primers: EstA part1 & EstA part2
- SOE PCR
- gene of interest: pNB-Est13
- primers: pNB-Est13 part1 & pNB-Est13 part2
- as a control a PCR is performed on EstA part2
- Annotation: SOE PCR is performed in 3 batches à 50 µL
- TA1 = 52°C, tA1 = 30 s, tE1 = 75 s
- TA2 = 57°C, tA2 = 30 s, tE2 = 75 s
- stored over night at 10°C
- qualitative Agarose gel electrophoresis
120801 SOE PCR Est13 EstA EstApart2 kontrolle.jpg
- 2 pNB-Est13, 3 ESTA, 4EstA part2
- PCR Clean up by Promega Kit of PhoA, pNB-Est13, EstA
- DNA Digestion of the following parts
Thursday, 02.08.12
- Ligation into digested and 5' dephosphorylated pSB1C3
- pNB-Est13
- PhoA
- EstA
- Annotation: Ligation is performed in a 20 µL batch
- digested pSB1C3 still availabe from Thursday, 19.07.12
- 1:5 & 1:10 ratio
- incubation at room temperature for 30 min
- Heatschock transformation of DH5alpha with our ligation batches
- incubation over night at 37°C
Friday, 03.08.12
- transformation succeeded
- colonies picked to inoculate 5 ml DYT-media-CM
Saturday, 04.08.12
- Miniprep of picked colonies
- test DNA Digestion with EcoRI-HF and PstI-HF
- PCR 2 on preped plasmids with primers VR & VF2, and the respective BBa primers
- TA = 60°C, ta = 35s, tE = 25 s
- qualitative Agarose gel electrophoresis 1% agarose
120804 Verdau PhoA, EstA, Est13, PCR auf EstA, Est 13, PhoA, Kontrolle auf Insert und Vektor.jpg
- digestion: 2 PhoA, 3 EstA, 4 pNB-Est13 PCR: 6 EstA with BBa primers, 7 EstA with VR & VF2, 8 pNB-Est13 with BBa primers, 10 pNB-Est13 with VR & VF2, 11 PhoA with BBa primers, 12 PhoA with VR & VF2
- tests succeeded
- Annotation: failure in mixing sequencing premixes
- retransformation of DH5alpha by Heatshock transformation with the following plasmids with 1 µL each
Week 4 / Kw 32
Assembly of our composite part RBS-PhoA-His6tag-pNBEst13-Myctag-EstA (BBa_K808030) is reached by synthesis (from GENEART) of 2 different gene parts:
- bba prefix-PhoA-His6tag-pNBEst13-BsaI site
- BsaI-Myctag-EstA- bba suffix
- the RBS will be added by PCR with primers XbaI Rbs Phoa up (ignoring the bba prefix but adding an XbaI site upstream of the RBS) & Est13 Bsa1 lo, ignoring the bba prefix but adding an XbaI site upstream of the RBS and for the downstream part primers EstA Bsa1 up andBBa EstA down are used
- both products can be assembled by using BsaI. An restriction enzyme, with an restriction site in a defined distance to its recognition site
Monday, 06.08.12
- Heatshock transformation of DH5alpha with 1 µL synthesis products (c=100 ng/µL):
- DNA Digestion of 10 µL = 1 µg of both synthesis products
- bba prefix-PhoA-His6tag-pNBEst13-BsaI site digested with EcoRI-HF and BsaI-HF in a 50 µL batch
- BsaI-Myctag-EstA- bba suffix digested with BsaI-HF and PstI-HF in a 50 µL batch
- preparative Agarose gel electrophoresis
- Failure due to overlooked additional PstI site in GENEART Vectors
- inoculation of 5 ml DYT-media-CAM with retransformations from Saturday, 04.08.12
- incubation at 37°C over night
Tuesday, 07.08.12
- Colony PCR on 4 colonies of each transformation of synthesis product from Monday, 06.08.12
- Annotation: PCR is done with house-taq, similar to PCR 2
- TA = 57°C, tA = 30 s, tE = 90 s
- Colony PCR succeeded
- from bba prefix-PhoA-His6tag-pNBEst13-BsaI site colonies 5, 2, 11 are picked
- from BsaI-Myctag-EstA- bba suffix colonies 1, 10, 16 are picked
- inoculation of 5 ml DYT-media-KAN with colonies
- incubation over night at 37°C
- inoculated 5 mL cultures from Monday, 06.08.12 are stored at 4°C
Wednesday, 08.08.12
- Miniprep of following 5 mL cultures
- from Monday, 06.08.12
- from Tuesday, 07.08.12
- bba prefix-PhoA-His6tag-pNBEst13-BsaI site
- colony 2 c=438 ng/µL
- colony 5 c=509 ng/µL
- colony 11 c=291 ng/µL
- BsaI-Myctag-EstA- bba suffix
- colony 1 c=381 ng/µL
- colony 10 c=420 ng/µL
- colony 16 c=263 ng/µL
- bba prefix-PhoA-His6tag-pNBEst13-BsaI site
- DNA Digestion
- of bba prefix-PhoA-His6tag-pNBEst13-BsaI site colony 11
- performed in a 40 µL batch
- BsaI and EcoRI are used
- digestion for 3 h
- of BsaI-Myctag-EstA- bba suffix
- performed in a 40 µL batch
- BsaI and SpeI are used
- digestion for 3 h
- of bba prefix-PhoA-His6tag-pNBEst13-BsaI site colony 11
- preperative Agarose gel electrophoresis
120808 Restriktion mit E BsaI PhoA Est13 und bsai s EstA.jpg
- 3-4 cut out bba prefix-PhoA-His6tag-pNBEst13-BsaI site, 5-6 cut out BsaI-Myctag-EstA- bba suffix
- Gel extraction
- c(bba prefix-PhoA-His6tag-pNBEst13-BsaI site)= 8 ng/µL
- c(BsaI-Myctag-EstA- bba suffix)= 26 ng/µL
- ligation of both inserts in pSB1C3 2 from Thursday, 19.07.12
- 1:5:5 ratio is performed in two 20 µL batches (one incubated at 4°C, the other at room temperature over night)
- new sequencing is ordered of the following plasmids
Thursday, 09.08.12
- Heatshock transformation of DH5alpha with 10 µL of each ligation batch from yesterday
Friday, 10.08.12
- transformation from yesterday suceeded
- one colony per plate (1:5:5 & 1:10:10 at room temperature and 1:5:5 & 1:10:10 at room 4°C)is picked for inoculation of 5 mL DYT-media-CAM
Saturday, 11.08.12
- Miniprep of picked colonies from yesterday
- testing ligation by DNA Digestion of plasmids with EcorI-HF & PstI-HF
- Colony PCR on picked colonies
- Ligation and transformation succeeded
120811 PCR (VR,VF2) und Verdau (EcoRI,PstI) auf BBa Gesamt von Geneart.jpg
- 2-5 bba prefix-PhoA-His6tag-pNBEst13-Myctag-EstA- bba suffix running at round about 3 kb
Week 5 / CW 33
Monday, 13.08.12
- Preparation for designing part BBa_K808032 which is an arabinose regulated RBS-PhoA-His6tag-pNBEst13-Myctag-EstA
- in order to add RBS upstream of PhoA of bba prefix-PhoA-His6tag-pNBEst13-BsaI site the following PCRs are performed
- PCR 1 on bba prefix-PhoA-His6tag-pNBEst13-BsaI site, as a template the pure synthesis product is used
- TA = 57°C, tA = 25 s, tE = 2 min
- primers: XbaI Rbs Phoa up & Est13 Bsa1 lo
- PCR 1 on BsaI-Myctag-EstA- bba suffix, as a template the pure synthesis product is used
- TA = 57°C, tA = 25 s, tE = 2 min
- primers: EstA Bsa1 lo & BBa EstA down
- PCR 3 on preped colony (1:5:5 room temperature from Friday, 10.08.12)
- TA = 57°C, tA = 25 s, tE = 2 min
- primers: XbaI Rbs Phoa up & BBa EstA down
- Annotation: each PCR is performed in 4 batches à 50 µL
- 2 qualitative Agarose gel electrophoresis
120813 PCR 1-4 RBSPE 5-8 EXN-Myc-Est 9-12.jpg
120813 PCR Q5 auf bba gesamt konstrukt.jpg
- 2-4 bba prefix-RBS-PhoA-His6tag-pNBEst13-Myctag-EstA-bba suffix
- PCRs succeeded
- PCR clean up of 1.-3. PCR
- DNA Digestion of pSB1C3 carrying RFP part ????
- DNA Digestion of part BBa_K808000 (pSB1C3 carrying Arabinose inducible promotor)
- preparative Agarose gel electrophoresis
- c(1.PCR)= 147 ng/µL
- c(2.PCR)= 133 ng/µL
- c(3.PCR)= 96 ng/µL
- c(cut S/P BBa_K808000: Arabinose inducible promotor) possibility 1= 14 ng/µL
- c(cut S/P BBa_K808000: Arabinose inducible promotor) possibility 2= 14 ng/µL
- c(cut X/P pSB1C3 carrying RFP)=20 ng/µL
- Ligation at 4°C over night of the following combinations:
- 1.PCR & 2.PCR in pSB1C3 carrying RFP
- 3.PCR in pSB1C3 carrying RFP
- 1.PCR & 2. PCR in BBa_K808000 possibility 1
- 3.PCR in BBa_K808000 possibility 1
- 1.PCR & 2. PCR in BBa_K808000 possibility 2
- 3.PCR in BBa_K808000 possibility 2
Tuesday, 14.08.12
- Heatshock transformation of ligation batches 1-6 from yesterday
Wednesday, 15.08.12
- transformation succeeded
- inoculation of 5 mL DYT-media-CAM of the following colonies
- colony 1.1
- colony 2.1
- colony 2.2
- colony 3.1
- colony 4.1
- colony 4.2
- colony 5.1
- colony 5.2
- colony 6.1
- colony 6.2
- incubation over night at 37°C
- Colony PCR on colonies 1.1-6.2 with house-taq
Thursday 16.08.12
- Miniprep of colonies from yesterday
- DNA Digestion of preped colonies with EcorI-HF & PstI-HF
- incubation for 1.5 h at 37°C
- qualitative Agarose gel electrophoresis
- FAILURE, because bands on gel are inconsistent
120816 Testrestriktion der pSB1C3 mit e und p colony platten 1 - 6.jpg
- 2 colony 1.1, 3 colony 2.1, 3 colony 2.2, 4 colony 3.1, 5 colony 4.1, 6 colony 4.2, 7 colony 5.1, 8 colony 5.2
- for further information see discussion below
- new inoculation of 5 mL DYT-media-CAM of Colonies 1.1 - 5.2
Trouble shooting
What should have been transformed:
- colony 1.1: 1.PCR & 2.PCR in pSB1C3
- should be RBS-PhoA-His6tag-pNBEst13-Myctag-EstA (BBa_K808030) in pSB1C3 (length: ~ 3.1 kb)
- colony 2.1: 3.PCR in pSB1C3 upstream of part BBa_K808000 (Arabinose inducible promotor) possibility 1
- should be Arabinose regulated RBS-PhoA-His6tag-pNBEst13-Myctag-EstA (BBa_K808032) in pSB1C3 (length: ~ 4.3kb)
- colony 2.2: similar to colony 2.1
- colony 3.1: 3.PCR in pSB1C3 upstream of part BBa_K808000 (Arabinose inducible promotor) possibility 2
- should be Arabinose regulated RBS-PhoA-His6tag-pNBEst13-Myctag-EstA (BBa_K808032) in pSB1C3 (length: ~ 4.3kb) like colony 2.1
- colony 4.1: 3.PCR in pSB1C3
- should be RBS-PhoA-His6tag-pNBEst13-Myctag-EstA (BBa_K808030) in pSB1C3 (length: ~ 3.1 kb)
- colony 4.2: similiar to colony 4.1
- colony 5.1: 1.PCR & 2.PCR in pSB1C3 upstream of part BBa_K808000 (Arabinose inducible promotor) possibility 1
- should be Arabinose regulated RBS-PhoA-His6tag-pNBEst13-Myctag-EstA (BBa_K808032) in pSB1C3 (length: ~ 4.3kb)
- colony 5.2: similar to colony 5.1
Conclusion:
- estimated gene length was aorund 4,3 kb (colony 5.1, 5.2, 2.1, 2.2 = part BBa_K808032) or 3,1 kb (colony 1.1, 4.1, 4.2 part BBa_K808030)
- digestion with EcoRI-HF & PstI-HF of all ligations from Thursday 16.08.12 in part BBa_K808000 (Arabinose inducible promotor) possibility 1 shows bands at round about 3 - 3.5 kb
- when digested with same enzymes of ligations into part BBa_K808000 (Arabinose inducible promotor) possibility 2, bands are shown at round about 1 - 1.5 kb
- this leads to our concluison that BBa_K808000 (Arabinose inducible promotor) possibility 1 was the not digested DNA band, because resulting gene length of cut insert correlates with length of BBa_K808000 (Arabinose inducible promotor)
- for a sharper solution an Agarose gel electrophoresis with 0.5 % agarose is performed for digestions of colonies 1.1, 3.1, 4.1, 4.2 plus batches from Colony PCR from Wednesday, 15.08.12
120816 Colony 1.1 3.1 4.1 4.2 Colony Pcr 1-8.tif
- 2-5 colonies 1.1, 3.1, 4.1, 4.2, 6 - 13 colony 1.1, 2.1, 2.2, 3.1, 4.1, 4.2, 5.1, 5.2
Friday, 17.08.12
- the image above means that:
- colony 5.1, 5.2, 2.1, 2.2 are only carrying part [[BBa_K808000 which encodes for the arabinosis inducable promotor
- but colony 1.1, 2.1, 2.2 are carrying 1.PCR & 2.PCR or 3.PCR in pSB1C3
- Miniprep of named colonies in liquid DYT-culture from Thursday 16.08.12
- half culture is preped, half culture serves for 10% DMSO stocks
- c(1.1: BBa_K808030)= 81 ng/µL
- c(4.1: BBa_K808030)= 128 ng/µL
- c(4.2: BBa_K808030)= 79 ng/µL
- c(2.1: BBa_K808000)= 90 ng/µL
- c(2.2: BBa_K808000)= 78 ng/µL
- c(5.1: BBa_K808000)= 64 ng/µL
- c(5.2: BBa_K808000)= 62 ng/µL
- for BioBrick assembly we perform a DNA Digestion of
- BBa_K808000 (colony 1.1, 4.1, 4.2), with EcoRI / SpeI, serves as upstream part
- BBa_K808030 (colony 5.1, 5.2, 2.1, 2.2), with EcoRI / XbaI, serves as downstream part
- preperative Agarose gel electrophoresis
- Gel extraction
- downstream part
- c(1.1: BBa_K808030)= 21.5 ng/µL
- c(4.1: BBa_K808030)= 54 ng/µL
- c(4.2: BBa_K808030)= 58 ng/µL
- upstream part
- c(2.1: BBa_K808000)= 12 ng/µL
- c(2.2: BBa_K808000)= 11 ng/µL
- c(5.1: BBa_K808000)= 10 ng/µL
- c(5.2: BBa_K808000)= 5 ng/µL
- ligation of upstream infron of downstream part
- colony 5.1 (BBa_K808000) in colony 1.1 (BBa_K808030)
- colony 2.2 (BBa_K808000) in colony 4.2 (BBa_K808030)
- colony 2.1 (BBa_K808000) in colony 4.1 (BBa_K808030)
- inoculation of 5 mL DYT-media-CAM of the following colonies
- colony 1.1
- colony 2.1
- colony 2.2
- colony 3.1
- colony 4.1
- colony 4.2
- colony 5.1
Week 6 / CW 34
Monday 20.08.12
- Miniprep of incoucaltes colonies from Friday, 17.08.12
- c(1.1: BBa_K808030)= 54 ng/µL
- c(4.1: BBa_K808030)= 74 ng/µL
- c(4.2: BBa_K808030)= 60 ng/µL
- c(2.1: BBa_K808000)= 47 ng/µL
- c(2.2: BBa_K808000)= 42 ng/µL
- c(5.1: BBa_K808000)= 56 ng/µL
- sequencing of
- colony 1.1 using VR & VF2, 1.5 µL each
- colony 5.1 using VR, SOE EstA mut lo, SOE EstA mut up & VF2, 1.5 µL each
Tuesday 21.08.12
- Ligation of BBa_K808000 upstream of BBa_K808032 in pSB1C3 ( to design BBa_K808032) from Friday, 17.08.12 worked well
- 6 colonies are picked in order to perform a Colony PCR and for inoculation of 5 mL DYT-media-CAM
- colony A 4.1/2.1 ligated at 4°C
- colony B 4.1/2.1 ligated at 37°C
- colony C 1.1/5.1 ligated at 4°C
- colony D 1.1/5.1 ligated at 37°c
- colony E 4.2/2.2 ligated at 4°C
- colony F 4.2/2.2 ligated at 37°C
- Colony PCR
- Agarose gel electrophoresis
- did not work due to immense gene legth ( around 4 kb)
- Restriction digest for testing is planned
- inoculation of 5 mL DYT-media-CAM with colony E,F,C,D
Wednesday, 22.08.12
- Miniprep of inoculated 5 mL DYT-media-CAM with colony E,F,C,D
- c(colony C 1.1/5.1 ligated at 4°C)=46 ng/µL
- c(colony D 1.1/5.1 ligated at 37°c)=46 ng/µL
- c(colony E 4.2/2.2 ligated at 4°C)=60 ng/µL
- c(colony F 4.2/2.2 ligated at 37°C)=58 ng/µL
- DNA Digestion of 15 µL of preped plasmids with EcoRI-HF & PstI-HF
- expected insert length: 4.4 kb (BBa_K808000+BBa_K808030)
- Agarose gel electrophoresis with 0.8% agarose
Verdau vom Gesamtkonstrukt 4.2_2.2 und 5.1_1.1 beide temperaturen.tif
- all bands are in same heights, so ligation worked well
- ligation worked well, bands are in estimated length
- due to rather bad results of Miniprep colonies are used for inoculation of 5 mL DYT-media-CAM again
- incubation over night at 37°C
- evaluation of sequencing from Wednesday, 08.08.12
- PhoA did not work
- EstA is sequenced inconsistent
- pNB-Est13 still got its illegal PstI-site
- but at leat FsC is sequenced completely
- new sequencing of
- PhoA in pSB1C3, primers VF2 & VR, 1.5 µL each
- EstA in pSB1C3, primers SOE EstA mut up & BBa EstA down, 1.5 µL each
- PCR 1 performed on BBa_K808032(preped colony C 1.1/5.1 ligated at 4°C)
- TA = 57°C, tA = 25 s, tE = 2 min
- primers BBa Est13 up & BBa Est13 down
- performed in 3 batches à 50 µL
- analytical Agarose gel electrophoresis 2% agarose
- [PCR Clean up]]
- DNA Digestion of PCR product with EcoRI-HF & PstI-HF over night at 37°C
Thursday, 23.09.12
- Miniprep of colonies from
- c(colony C 1.1/5.1 ligated at 4°C)=147 ng/µL
- c(colony D 1.1/5.1 ligated at 37°c)=131ng/µL
- c(colony E 4.2/2.2 ligated at 4°C)=85 ng/µL
- c(colony F 4.2/2.2 ligated at 37°C)=128 ng/µL
- preparative Agarose gel electrophoresis of digested pNB-Est13 from yesterday
- Gel extraction leads to c(pNB-Est13- cut E/P)=25 ng/µL
- Ligation of digested pNB-Est13 in digested pSB1C3
- 1:5 ratio is used
- Heatshock transformation of DH5alpha with 10 µL of ligation batch
- incubation of transformed cells at 37°C over night
Friday, 24.08.12
- transformation of ligation from yesterday succeeded, colony picked and inoculation of 5 mL DYT-media-CAM
- sequencing of
- BBa_K808032 (colony C 1.1/5.1 ligated at 4°C), primers VF2, XbaI Rbs Phoa up, BBa Ara Promo lo, SE Est13 mut up, SOE Est13 mut lo, BBa EstA down, VR, 1.5 µL each,
Week 7 / CW 35
Monday, 27.08.12
- Miniprep of picked colony from BBa_K808026 (pNB-Est13 in pSB1C3)
- sequencing of pNB-Est13 in pSB1C3
- using primers VF2, VR, SOE Est13 mut up, SOE Est13 mut lo
- test expression of BBa_K808032 (colony C 1.1/5.1 ligated at 4°C) in DH5alpha
- BBa_K808032 is an arabinose regulated RBS-PhoA-His6tag-pNBEst13-Myctag-EstA
- inoculation of 50 mL DYT-media-CAM with DH5alpha carrying BBa_K808032
- stop incubation at at OD600=0.7
- storaging cultures on ice for 15mins
- inducing with ~ 1.5% L-arabinose (using 3.75 mL of 20% mw L-arobinose solution)
- incubation at 20°C, 25°C and 30°C over night
Tuesday, 28.08.12
- antibody staining
- detection of surface expression of BBa_K808032 by using FACS
- positive signal increases with higher temperature, staining succeeded
- Protein ourification of test expression without rinnung IMAC afterwards, just using supernatant and cell debris pellet
- 32 µL of supernatant is used
- 3 mL pellet of each testexpression is treated with more SDS as a detergent, in order to solve membrane proteins
- running two SDS-tris gels with an myc positive probe
- one of these gels is used for performing a Westernblot
- using mouse-antimyc antibody as first antibody and goat-antimouse-AP as second antibody
120831 Westermblot auf Gesamt konstrukt aus DH5 alpha 5111.tif
- both are not very good in their solution, we do it again with Laemmli gels
Wednesday, 29.08.12
- evalutation of sequencing from last week:
- PhoA worked
- EstA worked
- pNB-Est13 worked
- RBS-PhoA-His6tag-pNBEst13-Myctag-EstA worked
- arabinose regulated RBS-PhoA-His6tag-pNBEst13-Myctag-EstA
Thursday, 30.08.12
- running 2 SDS-Laemmli gels
- pellet is treated with n-Dodecyl-ß-maltoside (DDM) in order to solve membrane proteins
- one gel is used to perform a second Western blot with a myc positive probe
120831 Westermblot 2 auf Gesamt konstrukt aus DH5 alpha 5111.tif
- 2 supernatant 30°C, 3 pellet (treated with detergent) 30°C, 4 supernatant 25°C, 5 pellet (treated with detergent) 25°C, 6 supernatant 20°C, 7 pellet (treated with detergent) 20°C, 8 myc positive probe
- as we can see, only the treated 120831 Westermblot 2 auf Gesamt konstrukt aus DH5 alpha 5111pellets show positive signals when treated with antibodies, so we have expressed our membrane protein BBa_K808032
- now our lab can start with quantification of enzymatic activites of BBa_K808032 on PET or model substrates
 "
"