Team:University College London/LabBook/Week5
From 2012.igem.org
Rwilkinson (Talk | contribs) (→Wednesday (11.7.12)) |
Rwilkinson (Talk | contribs) (→Tuesday 10.7.12) |
||
| Line 270: | Line 270: | ||
'''Results:''' The image below shows a 1% Agarose Gel of an Analytical Restriction Enzyme Digest for Expt 4.1. The products shown by the gel are all incorrect. In lane 1 we expected a product equivalent to the combined size of the BBA_K540000 insert and PSB1C3 backbone (uncut plasmid). The position of this is shown by A, which corresponds to 5193 bp. In Lane 2 and Lane 3, there are no products, though we had expected to see a band corresponding to the size of the BBa_K540000 insert (3123bp) as shown by B and a band corresponding to the size of the PSB1C3 backbone (2070bp) as shown by C. In Lane 4, there should be a product corresponding to the combined size of the BBa_I13522 insert and PSB1A3 backbone (3092bp) which is indicated by D. This is absent. In lane 5, there are numerous products, but none corresponding to the size of the BBa_I13522 insert (937bp) as indicated by E, or the PSB1A3 backbone (2155bp) as indicated by F. | '''Results:''' The image below shows a 1% Agarose Gel of an Analytical Restriction Enzyme Digest for Expt 4.1. The products shown by the gel are all incorrect. In lane 1 we expected a product equivalent to the combined size of the BBA_K540000 insert and PSB1C3 backbone (uncut plasmid). The position of this is shown by A, which corresponds to 5193 bp. In Lane 2 and Lane 3, there are no products, though we had expected to see a band corresponding to the size of the BBa_K540000 insert (3123bp) as shown by B and a band corresponding to the size of the PSB1C3 backbone (2070bp) as shown by C. In Lane 4, there should be a product corresponding to the combined size of the BBa_I13522 insert and PSB1A3 backbone (3092bp) which is indicated by D. This is absent. In lane 5, there are numerous products, but none corresponding to the size of the BBa_I13522 insert (937bp) as indicated by E, or the PSB1A3 backbone (2155bp) as indicated by F. | ||
| - | + | ||
| + | {{:Team:University_College_London/templates/pictureinsert|title=Picture 1|url=images/2/2b/UCLiGEM2012Gel_4.1.png}} | ||
'''Conclusion:''' It is likely the products are the result of contamination, and the transformation was a failure | '''Conclusion:''' It is likely the products are the result of contamination, and the transformation was a failure | ||
| Line 286: | Line 287: | ||
| BBa_KS40000 || 246.4 || 270.3 | | BBa_KS40000 || 246.4 || 270.3 | ||
|} | |} | ||
| - | |||
== Wednesday 11.7.12 == | == Wednesday 11.7.12 == | ||
Revision as of 17:36, 1 August 2012



Contents |
Monday (9.7.12)
Aim: Transformation of Key BioBricks
Method
(LOGO) Transformation Protocol 2
Step 1 – Thawing Cells: Use W3100 cell line created in Week 2 (Expt 2.1) Step 3 – Addition of BioBrick: To each 2ml eppendorf, add 1ul of the following BioBricks. Include an extra tube as a control, with no BioBrick added.
| Function | Module | ||
|---|---|---|---|
| BioBrick | BBa_K123003 | Oestrogen Receptor | Detection |
| BBa_K123002 | Oestrogen Response Element | Detection | |
| BBa_K398108 | Salt Tolerance Cluster | Salt Tolerance | |
| BBa_J23100 | Constitutive Promoter | ||
| BBa_J23107 | Constitutive Promoter | ||
| BBa_R0040 | TetR Repressible Promoter | Buoyancy | |
| BBa_J23106 | Constitutive Promoter | ||
| BBa_B0034 | Ribosome Binding Site (RBS) | All | |
| Control | (No BioBricks) | ||
Step 9 – Plating samples on Agar Plates: The table below indicates the chosen inoculation volume (two for each BioBrick) and the correct gel antibiotic concentration for all samples.
| Samples | Volume Inoculated | Antibiotic in Gel (ug/ml) | |
|---|---|---|---|
| BioBrick | BBa_ K123003 | 20ul | Ampicillin(50ug/ml) |
| 200ul | |||
| BBa_ K123002 | 20ul | ||
| 200ul | |||
| BBa_B0034 | 20ul | ||
| 200ul | |||
| BBa_J23100 | 20ul | ||
| 200ul | |||
| BBa_J23107 | 20ul | ||
| 200ul | |||
| BBa_R0040 | 20ul | ||
| 200ul | |||
| BBa_J23106 | 20ul | ||
| 200ul | |||
| BBa_ K398108 | 20ul | Chloramphenicol (25ug/ml) | |
| 200ul | |||
| Control | Positive (No BioBrick) | 36ul | No Antibiotic |
| Negative (No BioBrick) | 36ul | 2x Ampicillin(50ug/ml)
1x Chloramphenicol (25ug/ml) | |
Tuesday (10.7.12)
Aim - Check results from Transformation
Results: The table below indicates whether or not there was growth on each plate. Included is an image demonstrating the growth noted for BBa_K123003, BBa_K398108, and the Positive Control
| Samples | Volume Inoculated | Growth/No Growth | |
|---|---|---|---|
| BioBrick | BBa_ K123003 | 20ul | Growth |
| 200ul | Growth | ||
| BBa_ K123002 | 20ul | No Growth | |
| 200ul | No Growth | ||
| BBa_B0034 | 20ul | No Growth | |
| 200ul | No Growth | ||
| BBa_J23100 | 20ul | No Growth | |
| 200ul | No Growth | ||
| BBa_J23107 | 10ul | No Growth | |
| 2000ul | No Growth | ||
| BBa_R0040 | 20ul | No Growth | |
| 200ul | No Growth | ||
| BBa_J23106 | 20ul | No Growth | |
| 200ul | No Growth | ||
| BBa_ K398108 | 20ul | No Growth | |
| 200ul | Growth | ||
| Control | Positive (No BioBrick) | 36ul | Growth |
| Negative (No BioBrick) | 36ul | No Growth | |
Conclusion: Only BBa_K398108 and BBa_K123003 had successfully transformed. This is a serious setback, which suggests our cell competency is not as high as we first anticipated. Method
(LOGO) Picking Colonies
Step 2 - Inoculating Colonies into a Selective Broth: The table below indicates the volume of broth and the concentration of antibiotic used for the BioBrick.
| Samples | Volume Inoculated | Broth (ml) | Antibiotic (ug/ml) | |
|---|---|---|---|---|
| BioBrick | BBa_ K123003 | 20ul | Lysogeny Broth (5) | Ampicillin(50ug/ml) |
| 200ul | ||||
Wednesday (11.7.12)
Aim 1 – Check Results of Picking Colonies of BBa_K398108.
Result: The broth was cloudly, indicating there has been sufficient growth of bacteria to proceed to purification (miniprep) and an Analytical Restriction Digest to determine the presence of the correct BioBrick.
Method (LOGO) Miniprep Protocol 1 – Qiagen (LOGO) Restriction Digest
Step 2 – Setting up Digests and Controls: The protocol describes the recipe for (i) Digested Plasmid and (ii) Uncut Control. The table below indicates that an uncut and an Spe1/Xba1 digested sample be set up for each BioBrick.
| Samples | Recipe | Enzyme Used | Buffer | |
|---|---|---|---|---|
| BioBrick | BBa_K398108 | Digested Plasmid | Spe1 and Xba1 | 4 |
| Undigested Plasmid | None | |||
(LOGO) Gel Electrophoresis
Results: In Lane 1 and 2 we expect a product equivalent to the size of the PSB1C3 plasmid backbone and BBa_K398108 insert (2914bp) long, indicated by the position of A. The absence of this band, and the presence of other bands indicates that this transformation was unsuccessful. In Lane 3 and 4 we would expect a product for the BBa_K398108 insert (844bp) as indicated by B, and a product for the PSB1C3 plasmid backbone (2070bp) as indicated by C. Both products are absent, further supporting the failure of the transformation. In Lane 5 and 6 we would expect to see a similar product to Lanes 1 and 2, except for any secondary effect that the conformation of the uncut plasmid may have on its migration. Such a product was not found.

Conclusion: This transformation failed, or contamination has occurred since the colony picking.
(LOGO) Nanodrop
| BioBrick | 260 | 280 |
|---|---|---|
| BBa_K398108 | 173.8 | 186.6 |
Aim 2 - Picking Colonies for BBa_K123003. With the exception of BBa_K398108, which has already been analysed, BBa_K123003 was the only other BioBrick to show growth in the original Expt 5.1 cohort. Colonies from the Agar Plate will be selected and cultured, with the intent of purifying the plasmid for detecting (Restriction Enzyme Digest and Gel Electrophoresis) the presence of the correct BioBrick.
Method (LOGO) Picking Colonies
Step 2 - Inoculating Colonies into a Selective Broth: The table below indicates the volume of broth and the concentration of antibiotic used for inoculating the BioBrick.
| Samples | Volume Inoculated | Broth (ml) | Antibiotic (ug/ml) | |
|---|---|---|---|---|
| BioBrick | BBa_K123003 | 20ul | Lysogeny Broth (5) | Ampicillin(50ug/ml) |
| 200ul | ||||
Thursday 12.7.12
Aim 1 - Check results of picking colonies of BBa_K123003.
Results: There was no growth for the BioBrick.
Conclusion: The reasons for the lack of growth are not clear. As successfully transformed bacteria have already been selected for my the antibiotic positive agar plate, we would expect any colony picked to survive culture in broth of the same antibiotic concentration. It is possible that the colony did not make it into the Falcon but this is unlikely. We will repeat the picking of the BBa_K123003 colony.
Aim 2 – Picking Colonies of BBa_K123003: After we found the colony picking failed, we decided to repeat the protocol. This time however, we would make two inoculated falcons for each plate, to increase our chance of a successful culture.
Method: (LOGO) Picking Colonies
Step 2 - Inoculating Colonies into a Selective Broth: The table below indicates the volume of broth and the concentration of antibiotic used for inoculating the BioBrick.
| Samples | Volume Inoculated | Broth (ml) | Antibiotic (ug/ml) | |
|---|---|---|---|---|
| BioBrick | BBa_K123003 | 2x 20ul | Lysogeny Broth (5) | Ampicillin(50ug/ml) |
| 2x 200ul | ||||
Friday (13.7.12)
Aim 1 - Check results of Picking Colonies of BBa_K123003 (second attempt)
Results: There was growth for one falcon of BBa_Ki123003
Conclusion: Clearly BBa_K123003 is proving difficult to culture, but the presence of a single populated Falcon means we can proceed with purification (Miniprep).

Tuesday 10.7.12
Aim - Analytical Restriction Digest: This is intended to diagnose whether the correct BioBrick (BBa_I13522 and BBa_K540000) had been transformed into our bacteria, by measuring the size of the digested products against a DNA ladder.
Methods (LOGO) Restriction Digest
Step 2 – Setting up Digests and Controls: The protocol describes the recipe for (i) Digested Plasmid and (ii) Uncut Control. The table below indicates that an uncut and an EcoR1/Pst1 digested sample be set up for each BioBrick.
| Samples | Recipe | Enzyme Used! | |
|---|---|---|---|
| BioBrick | BBa_I13522 | Digested Plasmid | EcoR1 and Pst1 |
| Undigested Plasmid | None | ||
| BBa_K540000 | Digested Plasmid | EcoR1 and Pst1 | |
| Undigested Plasmid | None | ||
(LOGO) Gel Electrophoresis
Results: The image below shows a 1% Agarose Gel of an Analytical Restriction Enzyme Digest for Expt 4.1. The products shown by the gel are all incorrect. In lane 1 we expected a product equivalent to the combined size of the BBA_K540000 insert and PSB1C3 backbone (uncut plasmid). The position of this is shown by A, which corresponds to 5193 bp. In Lane 2 and Lane 3, there are no products, though we had expected to see a band corresponding to the size of the BBa_K540000 insert (3123bp) as shown by B and a band corresponding to the size of the PSB1C3 backbone (2070bp) as shown by C. In Lane 4, there should be a product corresponding to the combined size of the BBa_I13522 insert and PSB1A3 backbone (3092bp) which is indicated by D. This is absent. In lane 5, there are numerous products, but none corresponding to the size of the BBa_I13522 insert (937bp) as indicated by E, or the PSB1A3 backbone (2155bp) as indicated by F.
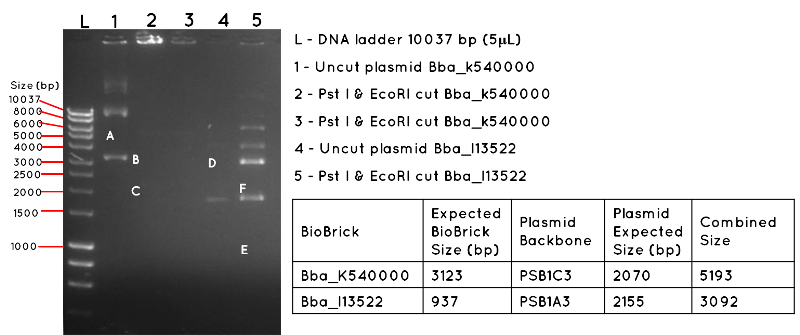
Conclusion: It is likely the products are the result of contamination, and the transformation was a failure
(LOGO) Nanodrop
Results: The table below indicates the concentrations indicated by the nanodropping for each plasmid.
| Header text | Concentration of plasmid (ng/ul) at wavelength 260 and 280 | |
|---|---|---|
| BioBrick | 260 | 280 |
| BBa_KS40000 | 246.4 | 270.3 |
Wednesday 11.7.12
Aim - Repeat Analytical Digest for Expt 4.1 using different enzymes: This is intended to test again whether the correct plasmid had been transformed into our bacteria, after the last gel produced unusual results. This time we will use a different pairs of enzymes, which
Results: The image below shows a 1% Agarose Gel of an Analytical Restriction Enzyme Digest for Expt 4.1. Lane 1 displays the products of a single cut reaction, which for the plasmid carrying BBa_K540000 should produce a product of 5193 bp – the sum of the plasmid backbone PSB1C3 and the BBa_K540000 insert. A very faint band corresponding to this size is indicated by A, but the presence of more prominant bands of incorrect size suggests this reaction failed. Lane 2 displays a product of the size corresponding to the BBa_K540000 insert (3123bp) as shown by B, and a product corresponding the PSB1C3 backbone (2070 bp) as shown by C. The larger product, labelled D, could be excess uncut plasmid, as it corresponds approximately to the correct size (5193bp). Lane 3 shows the products of a single cut reaction for BBa_I13522. A product corresponding to the combined size of BBa_I13522 insert and PSB1A3 plasmid backbone is present (3092bp) as indicated by E, but presence of other bands is concerning. Lane 4 shows an absence of the correct size insert (937 bp) as indicated by G, and an absence of plasmid backbone PSB1A3 (2155bp), as indicated by F.
(Insert gel)
Conclusion: This gel would indicate that the BBa_K540000 transformation was a success, but there is some concern about the presence of extra bands in Lanes 1 and 2. For this reason we will repeat this gel when possible (See Expt 7.2). The BBa_I13522 transformation appears to be unsuccessful – there is not band corresponding to the insert. The digest will be repeated to be certain (See Expt 7.2).

Thursday 12.7.12
Aim - Testing competency of Original W3100 Cell line: After the failure to transform the large majority of the cohort of Expt 5.1, and subsequent difficulties with culturing BBa_K123003, we are considering the possibility our W3100 cell line is not adequately competent. We are currently in the process of attempting a ‘reinvigoration’ of this cell line, but decided we should also do a formal test of the competency of the original W3100 cell line. To do so we will attempt to transform our W3100 cells with a plasmid (pTop) available from the lab, with a known concentration of 297ng/ul. At such a high concentration, transformation should be relatively easy.
If the transformation is successful, it could be the concentration of certain BioBricks (which is unknown) that is affecting the ability of the W3100s to undergo transformation. However, if transformation with pTop is not successful, it would suggest our cells are not competent.
Method
(LOGO) Transformation Protocol 2
Step 1 – Thawing Cells: Use W3100 cell line created in Week 2 (Expt 2.1)
Step 3 – Addition of BioBrick: To one 2ml eppendorf, add 1ul of pTop plasmid, and to another add nothing – this will be a control.
Step 7 – Adding Broth: Lysogeny Broth was used, as SOC media was not available.
Step 8 - Incubation: The table below indicates the Ampicillin concentration of the Agar gels. Cells cultured at 30oC over the weekend.
| Samples | Volume Inoculated | Antibiotic in Gel | |
|---|---|---|---|
| Plasmid | pTOP | 36ul | Ampicillin (50ug/ml) |
| Controls | Positive (No plasmid) | 36ul | No Antibiotic |
| Negative (No plasmid) | 36ul | Ampicillin (50ug/ml) | |

Monday (9.7.12)
Aim – Overnight Culture of Original line of W3100 cells: For the Reinvigoration we will be reapplying the protocol for Generating Competent Cells on the failed cell line. To do so, we must first inoculate the original cells into a falcon, and culture overnight.
Tuesday (10.7.12)
Aim – Day 1 of Generating Competent Cells: In order to culture our BioBricks in a cell line, we must first enable our cells to uptake plasmid. Today we will be starting the first day of the protocol, whereby we streak the cells on a minimal agar.
Method
(LOGO) – Day 1 of Generating Competent Cells
Wednesday (11.7.12)
Aim – Day 2 of Generating Competent Cells: Today we check the colony formation from yesterday’s culture, and pick a colony for inoculation
(LOGO) – Day 2 of Generating Competent Cells
Thursday (11.7.12)
Aim – Day 3 of Generating Competent Cells: Today do the final stages of the protocol, and should have a supply of competent cells.
(LOGO) – Day 3 of Generating Competent Cells
 "
"