Team:University College London/Module 3/Results
From 2012.igem.org
Sednanalien (Talk | contribs) (→Syringaldazine Assay of BBa_K729006 (Laccase)) |
Sednanalien (Talk | contribs) (→Syringaldazine Assay of BBa_K729006 (Laccase)) |
||
| Line 6: | Line 6: | ||
== Syringaldazine Assay of BBa_K729006 (Laccase) == | == Syringaldazine Assay of BBa_K729006 (Laccase) == | ||
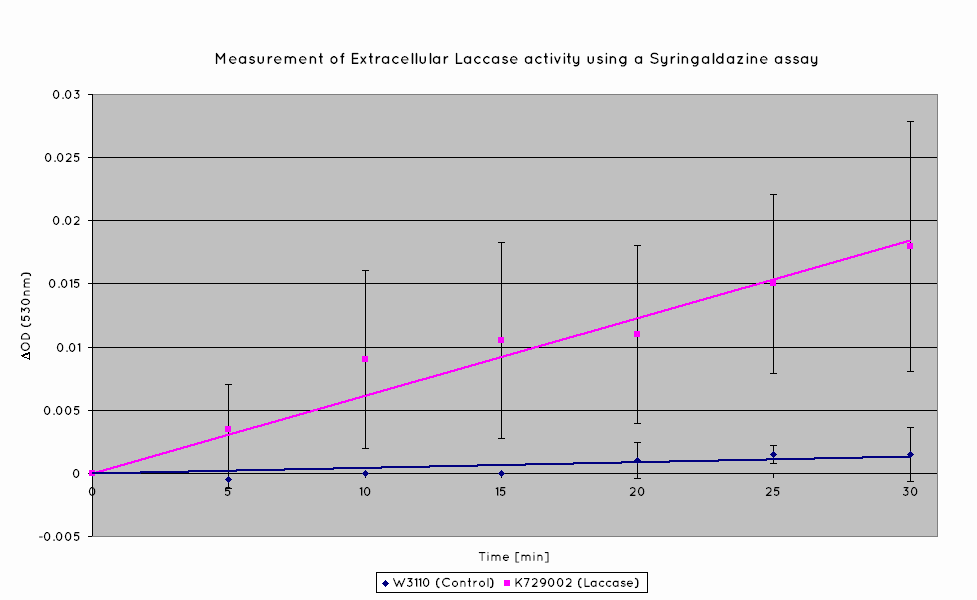
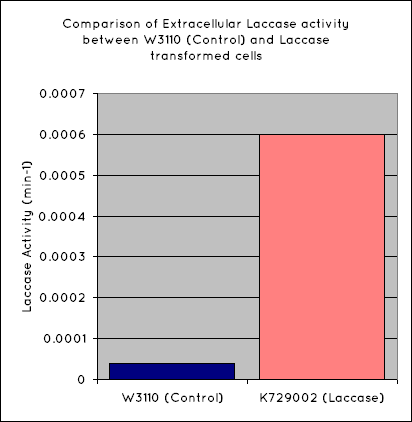
| - | The graphs below show the results of the Syringaldazine assay. Syringaldazine is oxidised by laccase in solution, causing a detectable change in optical density at 530nm. Our results indicate that a significantly higher rate of oxidation occurs for our BioBrick than the control. Quantitatively, this corresponds to a [?]- | + | The graphs below show the results of the Syringaldazine assay. Syringaldazine is oxidised by laccase in solution, causing a detectable change in optical density at 530nm. Our results indicate that a significantly higher rate of oxidation occurs for our BioBrick than the control. Quantitatively, this corresponds to a [?]-fold increase in laccase activity for our transformed cells than untransformed W3110. |
This indicates that our transformed ''E. coli'' have successfully produced laccase, allowing it to oxidise the syringaldazine utilised in the laccase assay. | This indicates that our transformed ''E. coli'' have successfully produced laccase, allowing it to oxidise the syringaldazine utilised in the laccase assay. | ||
Revision as of 09:43, 26 September 2012
Module 3: Degradation
Description | Design | Construction | Characterisation | Modelling | Results | Conclusions
Syringaldazine Assay of BBa_K729006 (Laccase)
The graphs below show the results of the Syringaldazine assay. Syringaldazine is oxidised by laccase in solution, causing a detectable change in optical density at 530nm. Our results indicate that a significantly higher rate of oxidation occurs for our BioBrick than the control. Quantitatively, this corresponds to a [?]-fold increase in laccase activity for our transformed cells than untransformed W3110.
This indicates that our transformed E. coli have successfully produced laccase, allowing it to oxidise the syringaldazine utilised in the laccase assay.
SEM for BBa_K729006 (Laccase)
The Scanning Electron Microscope (SEM) images below show the effects of subjecting Low Density Polyethylene (LDPE) to three different sets of conditions in order to visualise the degradative properties of cells containing our Laccase construct (BBa_K729006). We can then also compare this with the ability of untransformed E. coli W3110 and mechanical shear in water to breakdown the LDPE target.
All samples were in suspension at 37°C and 200rpm in an incubated shaker for 3 days.
Low magnification (x100) images were used to observe the macro-trend in zonal enzymatic activity. There appears to be increased ‘roughness’ (shown by the small protrusions on the surface as well as areas of darker shading, which represent areas of greater contour), across the surface of the plastic as we progress from the sample treated with water to untransformed E. coli W3110 to E. coli transformed with the UCL Laccase construct.
High magnification (x10 000) images were taken to give more high resolution information regarding the activity of the enzyme on a much smaller scale. The SEM images highlight increased amounts of ‘pitting’ (the generation of small holes in the plastic due to it’s structural breakdown), as we go from water to E. coli W3110 to bacteria containing BBa_K729006. These pits are shown by the much darker areas. This small scale breakdown has a cumulative effect which results in the overall trend seen at the lower magnification.
In conclusion, this collection of SEM images indicates an ability both of the BBa_K729006 transformed cells to produce the Laccase enzyme at greater titres than untransformed bacteria (though the E. coli W3110 is still seen to have some degradative effect), and of the Laccase produced to degrade the polyethylene target.
| Water | W3110 | Laccase | |
|---|---|---|---|
| Low Magnification (100x) | 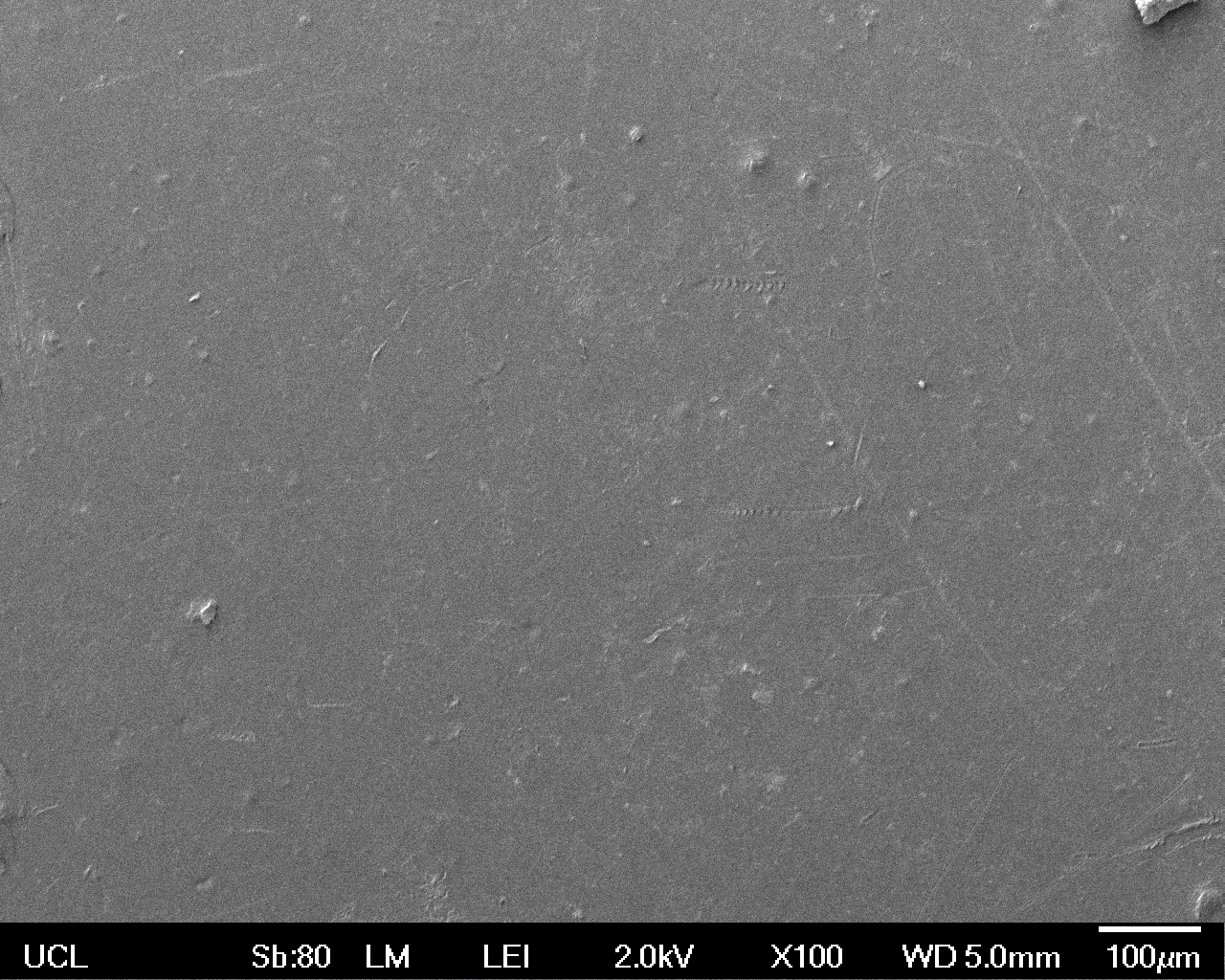 |  | 
|
| High Magnification (10 000x) | 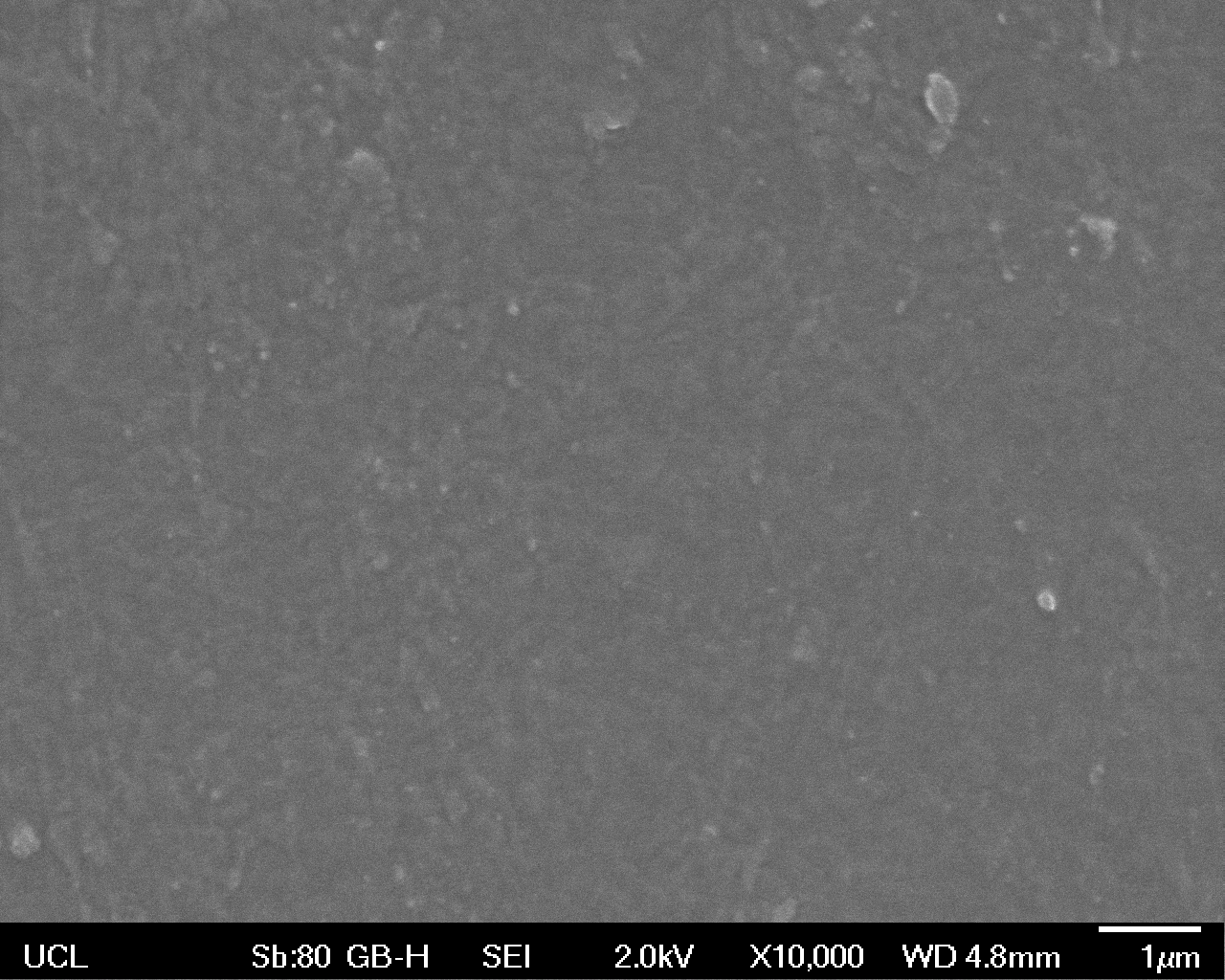 |  | 
|
 "
"