Team:University College London/Module 3/Modelling
From 2012.igem.org
Erinoerton (Talk | contribs) (→Sensitivity analysis) |
Erinoerton (Talk | contribs) (→Impact on experimental work) |
||
| Line 96: | Line 96: | ||
== Impact on experimental work == | == Impact on experimental work == | ||
| - | + | We initially believed that it was the amount of POPs present in the environment, rather than specific activity of the enzyme used, that would have the greatest effect on the amount of polyethylene degraded. Although we had identified <i>Rhodococcus ruber</i> strain C208 laccase (proven in a recent paper<sup>13</sup> to degrade polyethylene) as being a good potential laccase to use, we therefore decided to use a laccase which was easier to obtain (Ganoderma lucidum). | |
| + | |||
| + | Following the results of the sensitivity analysis, we realised that using a laccase with greater specific activity on PE would have a measurable impact on the amount of plastic our system might degrade. Future modifications to our system will use C208 laccase. | ||
== References == | == References == | ||
Revision as of 09:43, 25 September 2012
Contents |
Module 3: Degradation
Description | Design | Construction | Characterisation | Modelling | Results | Conclusions
Modelling
Building upon our earlier cell model for detection, our cell model for the degradation module shows the amount of laccase our bacteria produce and its action upon one type of microplastic present in the gyre, polyethylene. With this model we aimed to have an idea of how much plastic we could expect our system to degrade, which could then inform further predictive modelling around the amount of bacteria needed for Plastic Republic.
Our cell model was made in SimBiology. It shows the following reactions taking place in three 'compartments':
Outside1: in this compartment we see the association, adherence, and dissociation of persistent organic pollutants (POPs) from polyethylene (PE) (R1). We rely on the POPs to induce our degradation system, increasing (105 to 106 the specificity to our system.
Cell: NahR is a constitutively produced mRNA product (R9). When POP diffuses into the cell (R2), it forms a complex with NahR (R3) which then binds to the pSal promoter (R4) to induce production of laccase (R5, R6). Around 20% of this laccase diffuses outside of the cell (R7), the rest degrades (R10).
Outside2: in this compartment, the external laccase degrades PE (R9). Some of this external laccase is degraded (R11).
Species present in the cell model
| Species | Initial value (molecules) | Notes |
|---|---|---|
| PE | 0.044 | Polyethylene found in North Pacific Gyre (value per cubic metre)1,2 |
| POPex | 0.0 | Persistent organic pollutants (ex = extracellular) that are not adhered to plastic surface |
| PEPOPex | 9.24E-5 | Persistent organic pollutants (ex = extracellular) that are adhered to the plastic surface3 |
| POPin | 0.5 | Persistent organic pollutants (in = intracellular) assumed from E. coli membrane permeability 4 |
| mRNANahR | 0.0 | NahR mRNA product |
| POPinNahR | 0.0 | Complex of the above two molecules |
| POPinNahRpSal | 0.0 | Complex of the above molecule and pSal (promoter that induces laccase transcription) |
| Lin | 0.0 | Intracellular laccase |
| Lex | 0.0 | Extracellular laccase |
| LinmRNA | 0.0 | Laccase mRNA product |
| Ldegp | 0.0 | Laccase that degrades due to suboptimal conditions and malformed laccase that cannot carry out polyethylene degradation |
| PEdegp | 0.0 | Polyethylene degraded by laccase |
Reactions taking place in the model
| Number | Reaction | Reaction rate (molecules/sec) | Notes |
|---|---|---|---|
| R1 | PE + POPex ↔ PEPOPex | Forward: 10000 Backward: 1 | Pops have 10000 to 100000 times greater tendency to adhere to plastic than float free in the ocean5 |
| R2 | POPex ↔ POPin | Forward: 0.6 Backward: 0.4 | Based on membrane permeability4: diffusion gradient |
| R3 | POPin + mRNA.Nahr → POPin.mRNA.Nahr | Forward: 1 Backward: 0.0001 | Based on the assumption that the chemical structure/size of POPs is similar to salycilate6. Salycilate binds to the NahR mRNA product, which complex then binds to the pSal promoter. |
| R9 | 0 → mRNA.Nahr | Forward: 0.088 Backward: 0.6 | Transcription rate of NahR in molecules/sec (for NahR size 909 bp7, transcription rate in E.coli 80bp/sec8) under constitutive promoter control |
| R4 | POPinmRNANahr → POPinmRNANahr.Psal | Forward: 78200 Backward: 0.191 9 | NahR to pSal binding based on the assumption that POP-NahR binding has no effect on NahR-pSal binding |
| R5 | POPexmRNANahr.Psal → Lin.mRNA | 0.054 | Transcription rate of Laccase in molecules/sec (for laccase size 1500 bp10, transcription rate in E.coli 80bp/sec8) |
| R6 | Lin.mRNA → Lin | 0.04 | Translation rate of Laccase in molecules/sec (for laccase size 500 aa10, translation rate in E.coli 20aa/sec8) |
| R11 | Lin → Lindegp | 0.03 | Degradation rate of laccase11 must be taken into account due to suboptimal conditions |
| R7 | Lin → Lex | Forward: 0.9 Backward: 0.1 | Our laccase is a periplasmic enzyme, therefore most of it is released in the periplasm. However, we assumed leakage of 20 percent based on suboptimal conditions in the periplasm12, which is able to degrade polyethene. |
| R8 | Lex → PEdegp | Vm: 0.01 Km: 0.114 | Michaelis-Menten kinetics is used to represent degradation of polyethylene. As the literature values for the Km and Kcat for the polyethylene by laccase are not yet obtained experimentally for the original starin that was observed being able to degrade polyethylene13 we made an assumption that degradation of polyethylene is similar to that of lignin (due to polymeric nature of both), values for both Km and Kcat were taken from the literature14 |
Results
We ran three simulations in SimBiology, each over a different timespan:
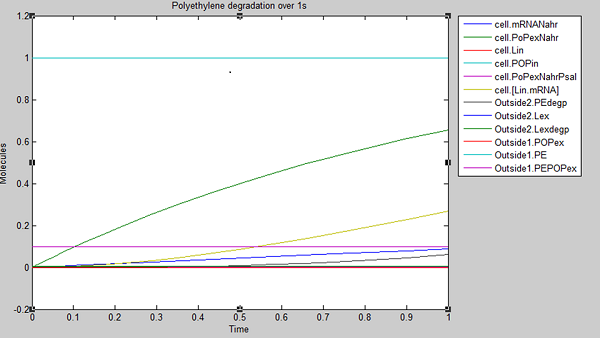 In the first second of laccase production, we see polyethylene degradation beginning from around 0.6 sec (Outside2.PEdegp)
In the first second of laccase production, we see polyethylene degradation beginning from around 0.6 sec (Outside2.PEdegp)
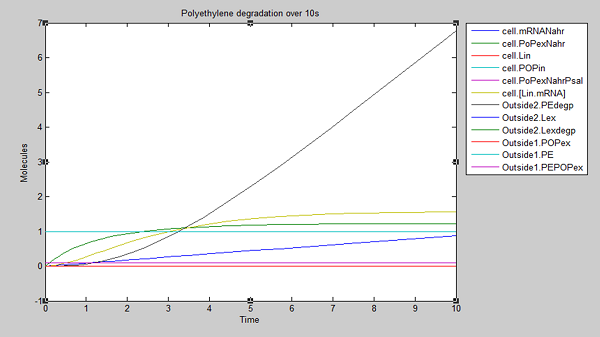 After 10 seconds, the first few molecules have been degraded. The constant diffusion of POPs into the cell is essential for the continuing degradation.
After 10 seconds, the first few molecules have been degraded. The constant diffusion of POPs into the cell is essential for the continuing degradation.
 At 100 seconds, the rate of degradation has risen to almost 1 PE molecule per second and we can assume that this will continue as long as the supply of POPs remains constant.
At 100 seconds, the rate of degradation has risen to almost 1 PE molecule per second and we can assume that this will continue as long as the supply of POPs remains constant.
From these results, we can see that our bacteria will degrade polyethylene. However, what these graphs can't show are the factors affecting degradation. For this reason, we decided to run a sensitivity analysis on our model.
Sensitivity analysis
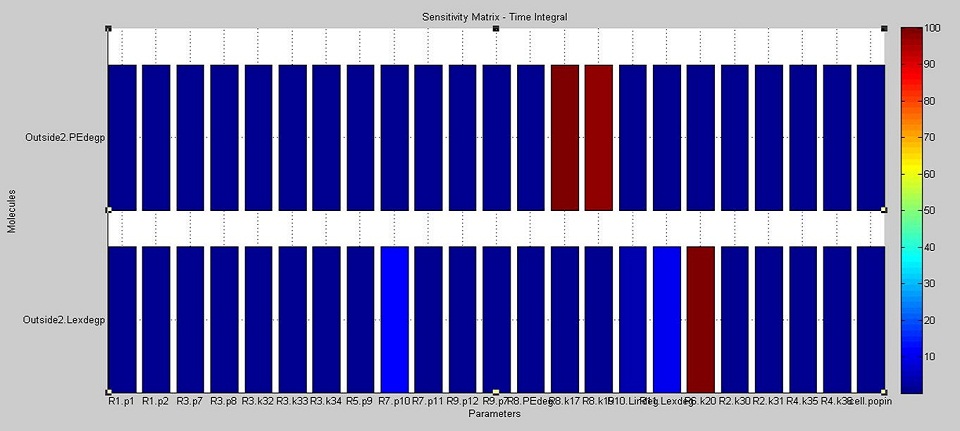
We used SimBiology's sensitivity analysis tool to compute sensitivities in our cell model. This tool examines the effect that each parameter (initially present species and reaction rates) has on the final concentrations of degraded PE and laccase.
The results show that the amount of PE degraded is most dependent on the kinetics of the laccase reaction (R8.k17 and R8.k19, the Vm and Km values respectively of our laccase). The amount of laccase degraded outside the cell is most dependent on the translation rate of laccase (R6.k20), but is also moderately affected by the rate of diffusion of laccase outside of the cell (R7.p10) and therefore on the amount of laccase which ends up outside of the cell (Lexdegp).
Impact on experimental work
We initially believed that it was the amount of POPs present in the environment, rather than specific activity of the enzyme used, that would have the greatest effect on the amount of polyethylene degraded. Although we had identified Rhodococcus ruber strain C208 laccase (proven in a recent paper13 to degrade polyethylene) as being a good potential laccase to use, we therefore decided to use a laccase which was easier to obtain (Ganoderma lucidum).
Following the results of the sensitivity analysis, we realised that using a laccase with greater specific activity on PE would have a measurable impact on the amount of plastic our system might degrade. Future modifications to our system will use C208 laccase.
References
1. Goldstein M, Rosenberg M, Cheng L (2012) Increased oceanic microplastic debris enhances oviposition in an endemic pelagic insect, Biology Letters 10.1098
2. Andrady AL (2011) Microplastics in the marine environment. Marine Pollution Bulletin 62: 1596-1605
3. To follow
4. To follow
5. Mato Y, Isobe T, Takada H, Kanehiro H, Ohtake C, Kaminuma T (2001) Plastic Resin Pellets as a Transport Medium for Toxic Chemicals in the Marine Environment. Environ. Sci. Technol. 35: 318-324
6. https://2011.igem.org/Team:Peking_S/project/wire/harvest
7. http://www.xbase.ac.uk/genome/azoarcus-sp-bh72/NC_008702/azo2419;nahR1/viewer
8. http://kirschner.med.harvard.edu/files/bionumbers/fundamentalBioNumbersHandout.pdf
9. Park H, Lim W, Shin H (2005) In vitro binding of purified NahR regulatory protein with promoter Psal. Biochimica et Biophysica Acta 1775: 247-255
10. Laccase size: http://partsregistry.org/Part:BBa_K729002
11. To follow
12. Young K, Silver LL (1991) Leakage of periplasmic enzymes from envA1 strains of Escherichia coli. J Bacteriol. 173: 3609–3614
13. Santo M, Weitsman R, Sivan A (2012) The role of the copper-binding enzyme - laccase - in the biodegradation of polyethylene by the actinomycete Rhodococcus ruber. International Biodeterioration & Biodegradation 208: 1-7
14. 5. Ding Z, Peng L, Chen Y, Zhang L, Gu Z, Shi G, Zhang K (2012) Production and characterization of thermostable laccase from the mushroom, Ganoderma lucidum, using submerged fermentation. African Journal of Microbiology Research 6: 1147-1157. DOI: 10.5897/AJMR11.1257
Misc
4. Teuten E, Saquing J, Knappe D et al. (2009) Transport and release of chemicals from plastics to the environment and to wildlife. Philosophical transactions of the Royal Society of London 364: 2027-2045
11.Van A, Rochman C, Flores E, Hill K, Vargas E, Vargas S, Hoh E (2012) Persistent organic pollutants in plastic marine debris found on beaches in San Diego, California. Chemosphere 86: 258-263
POPS & PLASTIC QUANTITITES
Rios L, Moore C, Jones P (2007) Persistent organic pollutants carried by synthetic polymers in the ocean environment. Marine Pollution Bulletin 54: 1230–1237
Takada H, et al (2010) Global distribution of organic micropollutants in marine plastics. Report to Algalita Marine Research Institute
Heskett M, Takada H, Yamashita R, Yuyama M, Ito M, Geok YB, Ogata Y, Kwan C, Heckhausen A, Taylor H, Powell T, Morishige C, Young D, Patterson H, Robertson B, Bailey E, Mermoz J (2012) Measurement of persistent organic pollutants (POPs) in plastic resin pellets from remote islands: Toward establishment of background concentrations for International Pellet Watch. Marine Pollution Bulletin 64: 445-448
6 Kunamneni A, Plou FJ, Ballesteros A, and Alcalde M. Laccases and their applications: A patent review. Departamento de Biocatálisis, Instituto de Catálisis y Petroleoquímica, CSIC, Cantoblanco, 28049 Madrid, Spain.
7. BRENDA: EC 1.10.3.2 – laccase
8. E.COLI MEMBRANE PERMEABILITY
Heskett M, Takada H, Yamashita R, Yuyama M, Ito M, Geok YB, Ogata Y, Kwan C, Heckhausen A, Taylor H, Powell T, Morishige C, Young D, Patterson H, Robertson B, Bailey E, Mermoz J (2012) Measurement of persistent organic pollutants (POPs) in plastic resin pellets from remote islands: Toward establishment of background concentrations for International Pellet Watch. Marine Pollution Bulletin 64: 445-448 is this right? This is what’s on excel
7. DIFFERENT REGULATOR SYSTEMS Wang B, Papamichail D, Mueller S, Skiena S (2007) Two Proteins for the Price of One: The Design of Maximally Compressed Coding Sequences. DOI: 10.1.1.174.6416
 "
"