Team:HokkaidoU Japan/Project/Biocapsule
From 2012.igem.org
(→Method) |
(→precipitation test) |
||
| (44 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
<div id="hokkaidou-column-main"> | <div id="hokkaidou-column-main"> | ||
<!-- DO NOT EDIT OVER THIS LINE @iTakeshi --> | <!-- DO NOT EDIT OVER THIS LINE @iTakeshi --> | ||
| - | |||
| - | |||
==Introduction== | ==Introduction== | ||
| - | + | <div class="hokkaidou-section"> | |
<p> | <p> | ||
The aim of our project was to make a smart system for industrial production of high-value added macromolecules by using BioDevice function in E. coli. We call it, “Bio capsule” method. At the beginning, we expected to be able to make “Bio-capsule” only by employing a function of “Aggregation module”, however, resultant E. coli cluster was not sufficiently hard enough to be recovered on filter through nylon mesh filtration. However we accidentally found that co-expression of both “Plastic Producing Module” and “Aggregation Module” results in forming hard and heavy E. coli clusters which can be called real “Bio capsule” we expected. | The aim of our project was to make a smart system for industrial production of high-value added macromolecules by using BioDevice function in E. coli. We call it, “Bio capsule” method. At the beginning, we expected to be able to make “Bio-capsule” only by employing a function of “Aggregation module”, however, resultant E. coli cluster was not sufficiently hard enough to be recovered on filter through nylon mesh filtration. However we accidentally found that co-expression of both “Plastic Producing Module” and “Aggregation Module” results in forming hard and heavy E. coli clusters which can be called real “Bio capsule” we expected. | ||
</p> | </p> | ||
| - | + | </div> | |
==Method== | ==Method== | ||
| - | + | <div class="hokkaidou-section"> | |
<p> | <p> | ||
We used two kinds of compatible plasmid vector to make E. coli expresses both Ag43 and all enzymes required for P3HB production. We chose pSB1C3 plasmid vector, a high copy number plasmid vector containing replication origin from R- factor, for the expression of “Plastic Producing Module” to produce enough amount of bio-plastic. We chose pSTV28 plasmid vector that contains compatible replication origin to pSB series: the most popular plasmid vector in iGEM, for expression “Aggregation Module”. It is widely known that if there were two similar plasmids those containing same replication origin in single cell, they compete with each other for replication and only one of which can be amplified in E. coli. <br /> | We used two kinds of compatible plasmid vector to make E. coli expresses both Ag43 and all enzymes required for P3HB production. We chose pSB1C3 plasmid vector, a high copy number plasmid vector containing replication origin from R- factor, for the expression of “Plastic Producing Module” to produce enough amount of bio-plastic. We chose pSTV28 plasmid vector that contains compatible replication origin to pSB series: the most popular plasmid vector in iGEM, for expression “Aggregation Module”. It is widely known that if there were two similar plasmids those containing same replication origin in single cell, they compete with each other for replication and only one of which can be amplified in E. coli. <br /> | ||
| - | + | [[image:HokkaidoU Bio capsule module.2.png|center|thumb|650px|Fig. 1 Image of our Bio capsule]] | |
| - | + | As a pilot experiment, we made E. coli containing plasmid for “Aggregation module” and plasmid for “Plastic producing module”. “Aggregation module” is induced by L-arabinose, since Ag43 is expressed under control of PBAD promoter. “Plastic producing module” used in the experiment in table 1 and 2 is expressed under control of original promoter of gene cluster encoding all enzymes required for P3HB production in R. eutropha. | |
| - | [[image:HokkaidoU Bio capsule module.2.png|center|thumb| | + | |
| - | + | ||
| - | + | ||
| - | As a pilot experiment, we made E. coli containing plasmid for “Aggregation module” and plasmid for “Plastic producing module”. “Aggregation module” is induced by L-arabinose, since Ag43 is expressed under control of PBAD promoter. “Plastic producing module” used in the experiment in table 1 and 2 is expressed under control of original promoter of gene cluster encoding all enzymes required for P3HB production in R. eutropha. | + | |
| - | + | ||
| - | + | ||
</p> | </p> | ||
| + | </div> | ||
==Results== | ==Results== | ||
| + | <div class="hokkaidou-section"> | ||
<p> | <p> | ||
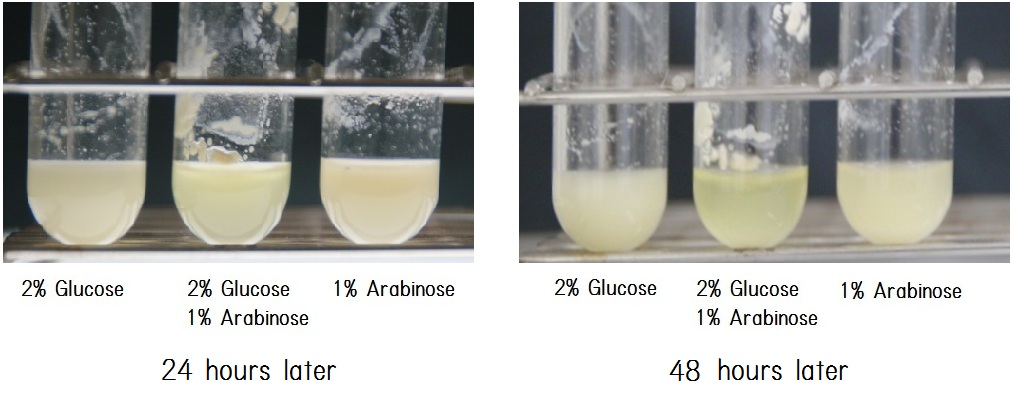
| - | The aggregation is induced by the addition of arabinose for 1%, and production of P3HB is initiated by the addition of glucose for 2% as energy source, so adding both chemicals to the culture media initiates to start expression of both modules. Hard and heavy E. coli clusters were observed only in a presence of these chemicals as shown in | + | The aggregation is induced by the addition of arabinose for 1%, and production of P3HB is initiated by the addition of glucose for 2% as energy source, so adding both chemicals to the culture media initiates to start expression of both modules. Hard and heavy E. coli clusters were observed only in a presence of these chemicals as shown in Table1.</p> |
| - | [[image: | + | [[image:HokkaidoU Table1.jpg|center|thumb|650px|Table1]] |
| - | + | [[image:HokkaidoU2012_24h-48h.jpg|center|thumb|650px|fig. 2]] | |
| - | + | <p> | |
| - | [[image:HokkaidoU2012_24h-48h.jpg|center|thumb| | + | Fig. 2 shows photographic image of the culture tested. Each condition is indicated below each image. After cultivation for 24hours, each three culture media did not have big difference (panel A in fig. 2). However, after cultivation for 48hours, we could clearly observe that in the second sample (2%glucose (+) 1%Arabinose (+)), its supernatant had high clarity than others (panel B in fig. 2). <br /> |
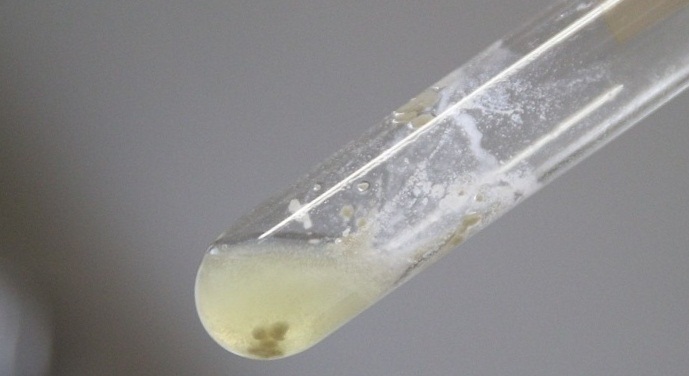
| - | + | Furthermore, hard and heavy clusters of cells were sticking to the surface of side wall in the test tube and precipitating in the media (fig. 3). | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
</p> | </p> | ||
| - | + | [[image:HokkaidoU IMG 2463.JPG|center|thumb|650px|Fig. 3 clustered cells]] | |
| - | ===precipitation test=== | + | <div style="width: 100%; height: 30px;"></div> |
| - | [[image: | + | ====precipitation test==== |
| - | + | [[image:Hokkaido Uver20min & 5min.jpg|center|thumb|500px|0min & 5min]] | |
[[image:HokkaidoU10min &15min.jpg|center|thumb|500px|10min & 15min]] | [[image:HokkaidoU10min &15min.jpg|center|thumb|500px|10min & 15min]] | ||
| - | + | [[image:HokkaidoU30min & 1hr.jpg|center|thumb|500px|30min & 1hr]] | |
| - | + | ||
| - | [[image:HokkaidoU30min & 1hr.jpg|center|thumb|500px|30min & | + | |
<p> | <p> | ||
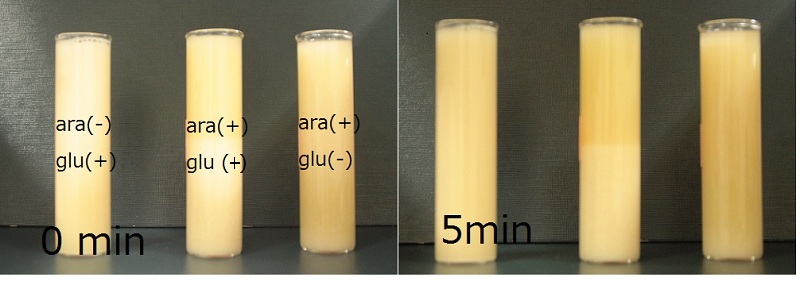
The speed of precipitation was measured. | The speed of precipitation was measured. | ||
| + | We cultured "bio plastic module" and "Aggreration module" transformed E. coli in the scale of 50ml(Table1). | ||
The photograph of media was taken at the timing of 0min, 5min, 10min, 15min, 30min, and 1hr. | The photograph of media was taken at the timing of 0min, 5min, 10min, 15min, 30min, and 1hr. | ||
| + | As you can see, the media with the expression of both Ag43 and phaCAB, had the fastest precipitation. | ||
| - | + | </p> | |
| + | <div style="width: 100%; height: 30px;"></div> | ||
| + | ====Staining by Nile red==== | ||
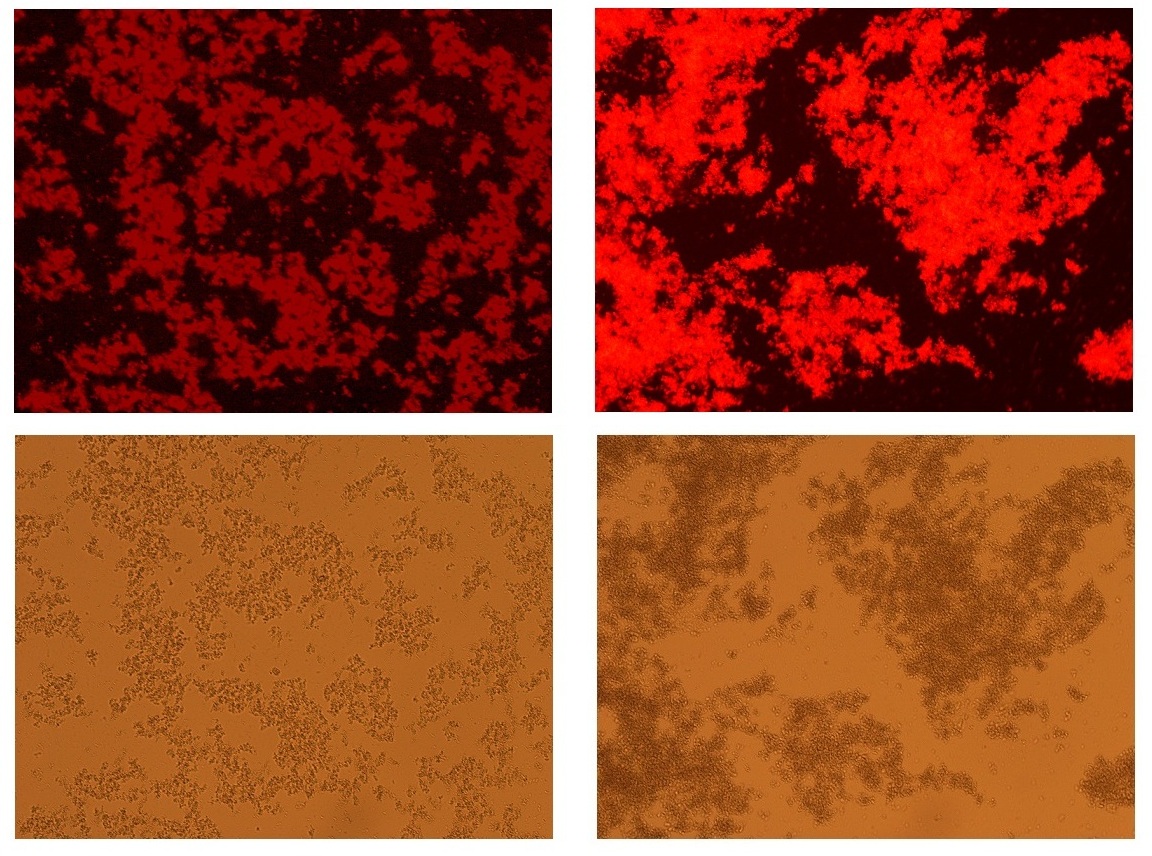
| + | <p> | ||
| + | We identified the production of plastic by conventional staining method (staining with Nile red) in E. coli containing both "plastic producing module" and "Aggregation module". | ||
| + | Specific signal associated with interaction between Nile red and P3HB was detected only in the presence of plastic producing module(right panel above), but not in the absence of it(left panel above) | ||
| + | </p> | ||
| + | [[image:HokkaidoU2012_Nilered.jpg|center|thumb|600px|Staining with Nile red (Fluorescenced photograph above)]] | ||
| + | <div style="width: 100%; height: 30px;"></div> | ||
| + | ====HPLC test==== | ||
| + | <p> | ||
| + | We confirmed the existence of P3HB by analyzing building block of it after hydrolysis of P3HB by using HPLC. | ||
| + | Degraded building block of P3HB was detected in E. coli containing both "plastic producing module" and "Aggregation module". | ||
</p> | </p> | ||
| + | [[image:pGEM+Ag (A G AG).jpg|center|thumb|350px|Result of HPLC test, A=added only arabinose G=added only glucose AG=addded both arabinose and glucose.]] | ||
| + | </div> | ||
==Discussion(Improvement)== | ==Discussion(Improvement)== | ||
| + | <div class="hokkaidou-section"> | ||
<p> | <p> | ||
Our finding of method to create “Hard and heavy cluster” when both Ag43 and phaCAB was expressed, was an unexpected result. However, this result we got was a positive one, and we can say it is our team’s serendipity. | Our finding of method to create “Hard and heavy cluster” when both Ag43 and phaCAB was expressed, was an unexpected result. However, this result we got was a positive one, and we can say it is our team’s serendipity. | ||
| - | When we expressed Ag43 only, the cell makes a weak cluster which is not suitable for harvesting “Plastic producing module”. However, when we combine two modules intoa single cell, and induce both of their expressions, the cells produces “Hard and heavy cluster” which fits for our image of “Bio-capsule”. | + | When we expressed Ag43 only, the cell makes a weak cluster which is not suitable for harvesting “Plastic producing module”. However, when we combine two modules intoa single cell, and induce both of their expressions, the cells produces “Hard and heavy cluster” which fits for our image of “Bio-capsule”. We estimate that the expression of plastics makes the cells heavier than wild type E. coli and results to precipitate the cells faster than ever. In addition, the plastic have acted as a glue to reinforce the attachment of Ag43 protein locating on the surface of E. coli cells.(Fig .4) |
</p> | </p> | ||
| + | [[image:HokkaidoU Ag43+PHB もっと強くなる!.png|center|thumb|1000px|Fig. 4 image]] | ||
| + | </div> | ||
==Future planning== | ==Future planning== | ||
| + | <div class="hokkaidou-section"> | ||
<p> | <p> | ||
| - | + | Our result indicates the success of collecting cells by filtration or by decantation in large scales like manufacturing. When manufacturing bio plastics by bacteria, the method of harvesting is indispensable. However, the method of centrifugation is time and cost consuming, especially when the scale gets larger. In contrast, with the usage with our bio capsule module, the method of harvesting turns into an easy and low costing job. Our success may contribute to future manufacturing’s bio plastics by bacteria. | |
In addition, the candidate inside of the bio capsule is not limited to P3HB. We have various kinds of plastics that are useful. For example, P4HB(4-Hydroxybutyrate) described as a strong pliable thermoplastic material is used for surgery strings because it is elongate and bio degradable. Furthermore, P4HB could form co-polymers with P3HB. If we succeed to submit the enzymes relating to the synthesis of P4HB, we will be able to construct an bio capsule which contains P4HB. | In addition, the candidate inside of the bio capsule is not limited to P3HB. We have various kinds of plastics that are useful. For example, P4HB(4-Hydroxybutyrate) described as a strong pliable thermoplastic material is used for surgery strings because it is elongate and bio degradable. Furthermore, P4HB could form co-polymers with P3HB. If we succeed to submit the enzymes relating to the synthesis of P4HB, we will be able to construct an bio capsule which contains P4HB. | ||
</p> | </p> | ||
| - | + | </div> | |
| - | + | ||
<!-- DO NOT EDIT UNDER THIS LINE @iTakeshi --> | <!-- DO NOT EDIT UNDER THIS LINE @iTakeshi --> | ||
</div> | </div> | ||
<br style="clear: both; line-height: 0;" /> | <br style="clear: both; line-height: 0;" /> | ||
{{Team:HokkaidoU_Japan/footer}} | {{Team:HokkaidoU_Japan/footer}} | ||
Latest revision as of 03:58, 27 September 2012
Contents |
Introduction
The aim of our project was to make a smart system for industrial production of high-value added macromolecules by using BioDevice function in E. coli. We call it, “Bio capsule” method. At the beginning, we expected to be able to make “Bio-capsule” only by employing a function of “Aggregation module”, however, resultant E. coli cluster was not sufficiently hard enough to be recovered on filter through nylon mesh filtration. However we accidentally found that co-expression of both “Plastic Producing Module” and “Aggregation Module” results in forming hard and heavy E. coli clusters which can be called real “Bio capsule” we expected.
Method
We used two kinds of compatible plasmid vector to make E. coli expresses both Ag43 and all enzymes required for P3HB production. We chose pSB1C3 plasmid vector, a high copy number plasmid vector containing replication origin from R- factor, for the expression of “Plastic Producing Module” to produce enough amount of bio-plastic. We chose pSTV28 plasmid vector that contains compatible replication origin to pSB series: the most popular plasmid vector in iGEM, for expression “Aggregation Module”. It is widely known that if there were two similar plasmids those containing same replication origin in single cell, they compete with each other for replication and only one of which can be amplified in E. coli.
As a pilot experiment, we made E. coli containing plasmid for “Aggregation module” and plasmid for “Plastic producing module”. “Aggregation module” is induced by L-arabinose, since Ag43 is expressed under control of PBAD promoter. “Plastic producing module” used in the experiment in table 1 and 2 is expressed under control of original promoter of gene cluster encoding all enzymes required for P3HB production in R. eutropha.
Results
The aggregation is induced by the addition of arabinose for 1%, and production of P3HB is initiated by the addition of glucose for 2% as energy source, so adding both chemicals to the culture media initiates to start expression of both modules. Hard and heavy E. coli clusters were observed only in a presence of these chemicals as shown in Table1.
Fig. 2 shows photographic image of the culture tested. Each condition is indicated below each image. After cultivation for 24hours, each three culture media did not have big difference (panel A in fig. 2). However, after cultivation for 48hours, we could clearly observe that in the second sample (2%glucose (+) 1%Arabinose (+)), its supernatant had high clarity than others (panel B in fig. 2).
Furthermore, hard and heavy clusters of cells were sticking to the surface of side wall in the test tube and precipitating in the media (fig. 3).
precipitation test
The speed of precipitation was measured. We cultured "bio plastic module" and "Aggreration module" transformed E. coli in the scale of 50ml(Table1). The photograph of media was taken at the timing of 0min, 5min, 10min, 15min, 30min, and 1hr. As you can see, the media with the expression of both Ag43 and phaCAB, had the fastest precipitation.
Staining by Nile red
We identified the production of plastic by conventional staining method (staining with Nile red) in E. coli containing both "plastic producing module" and "Aggregation module". Specific signal associated with interaction between Nile red and P3HB was detected only in the presence of plastic producing module(right panel above), but not in the absence of it(left panel above)
HPLC test
We confirmed the existence of P3HB by analyzing building block of it after hydrolysis of P3HB by using HPLC. Degraded building block of P3HB was detected in E. coli containing both "plastic producing module" and "Aggregation module".
Discussion(Improvement)
Our finding of method to create “Hard and heavy cluster” when both Ag43 and phaCAB was expressed, was an unexpected result. However, this result we got was a positive one, and we can say it is our team’s serendipity. When we expressed Ag43 only, the cell makes a weak cluster which is not suitable for harvesting “Plastic producing module”. However, when we combine two modules intoa single cell, and induce both of their expressions, the cells produces “Hard and heavy cluster” which fits for our image of “Bio-capsule”. We estimate that the expression of plastics makes the cells heavier than wild type E. coli and results to precipitate the cells faster than ever. In addition, the plastic have acted as a glue to reinforce the attachment of Ag43 protein locating on the surface of E. coli cells.(Fig .4)
Future planning
Our result indicates the success of collecting cells by filtration or by decantation in large scales like manufacturing. When manufacturing bio plastics by bacteria, the method of harvesting is indispensable. However, the method of centrifugation is time and cost consuming, especially when the scale gets larger. In contrast, with the usage with our bio capsule module, the method of harvesting turns into an easy and low costing job. Our success may contribute to future manufacturing’s bio plastics by bacteria. In addition, the candidate inside of the bio capsule is not limited to P3HB. We have various kinds of plastics that are useful. For example, P4HB(4-Hydroxybutyrate) described as a strong pliable thermoplastic material is used for surgery strings because it is elongate and bio degradable. Furthermore, P4HB could form co-polymers with P3HB. If we succeed to submit the enzymes relating to the synthesis of P4HB, we will be able to construct an bio capsule which contains P4HB.
 "
"