Team:HokkaidoU Japan/Notebook/aggregation Week 4
From 2012.igem.org
(→Electrophoresis) |
|||
| (55 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
<!-- DO NOT EDIT OVER THIS LINE @iTakeshi --> | <!-- DO NOT EDIT OVER THIS LINE @iTakeshi --> | ||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==July | + | ===July 23rd=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | == | + | ====Plasmid extraction==== |
| - | + | Plasmid extraction for Ag43(resuspended colony incubated at July 17th and resuspended colony incubated at July 20th). We used FastGene Plasmid Mini Kit(Nippon Genetics) and got 50 ul of DNA solutions. | |
| - | + | ||
| - | + | ||
| + | <br style="line-height: 0; clear: both;" /> | ||
</div></div> | </div></div> | ||
| + | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==July 24th== | + | ===July 24th=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | ==Electrophoresis== | + | =====Electrophoresis===== |
| - | + | Electrophoresis for Ag43(plasmid extraction at July 23rd) and Ag43 digestion results(digested with EcoRI and SpeI) | |
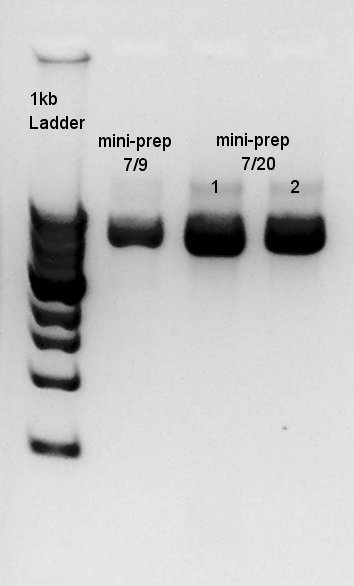
| - | Electrophoresis for Ag43( | + | [[image:HokkaidoU2012 120724 ag43-dt pcr.jpg|thumb|Plasmid extraction result]] |
| - | + | ||
| - | [[image:HokkaidoU2012 120724 ag43-dt pcr.jpg|thumb| | + | |
| - | + | ||
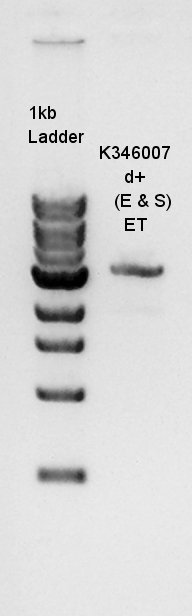
[[image:HokkaidoU2012 120724 Ag43(K346007 cut with E&S) digestion product.jpg|thumb|Digestion result]] | [[image:HokkaidoU2012 120724 Ag43(K346007 cut with E&S) digestion product.jpg|thumb|Digestion result]] | ||
| + | From this digestion result, we found out that one or two enzymes didn't work successfully but there are enough concentration of DNA in 3000bp band to use for digestion. | ||
| - | + | ====Gel extraction==== | |
| + | Gel extraction for digestion production. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution. | ||
| - | + | ====Ethanol precipitation==== | |
| - | == | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | ==Ethanol precipitation== | + | |
| - | + | ||
Ethanol precipitation for digestion and gel extraction product. | Ethanol precipitation for digestion and gel extraction product. | ||
| - | #Added | + | #Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. |
| - | #Centrifuged | + | #Centrifuged at 15000 rpm, 10min at 4C. |
| - | # | + | #Removed supernatant and added 220 ul of 70% ethanol. |
| - | #Centrifuged | + | #Centrifuged at 15000 rpm, 10 min at 4C. |
| - | # | + | #Removed supernatant and dried out at room temperature after that added 10 ul of DW. |
[[image:HokkaidoU2012 120724 Ag43(K346007 cut with E&S) digestion product ethanol precipitation.jpg|thumb|Ethanol precipitation result]] | [[image:HokkaidoU2012 120724 Ag43(K346007 cut with E&S) digestion product ethanol precipitation.jpg|thumb|Ethanol precipitation result]] | ||
| - | + | From this result, we estimated that the concentration of ethanol precipitation product is about 40 ng/ul. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | ====Digestion==== | ||
| + | Digestion to confirm how many PstI cutting sites are there in K346007 and for Ag43-dT complex with SpeI and XbaI. | ||
| + | <br /> | ||
Ag43 | Ag43 | ||
PstI | PstI | ||
| Line 54: | Line 44: | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |5 ul |
|- | |- | ||
|PstI | |PstI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |12 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |20 ul |
|} | |} | ||
| - | Ag43-dT | + | Ag43-dT<br /> |
SpeI and XbaI | SpeI and XbaI | ||
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |12 ul |
|- | |- | ||
|SpeI | |SpeI | ||
| - | | | + | |1 ul |
|- | |- | ||
|XbaI | |XbaI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |4 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |20 ul |
|} | |} | ||
| - | + | ||
| - | ==Electrophoresis== | + | ====Electrophoresis==== |
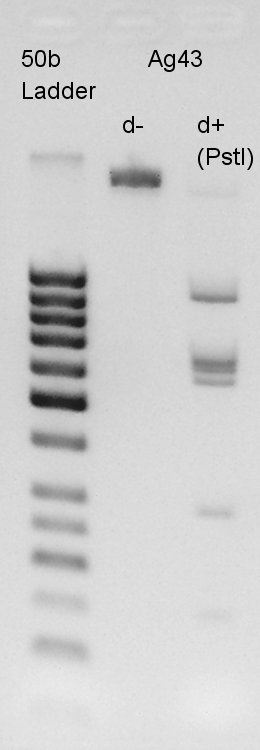
| - | + | [[image:HokkaidoU2012 120724 Ag43(K346007 cut with E&S then cut with P).jpg|thumb|Ag43 d+(P) Digestion result]] | |
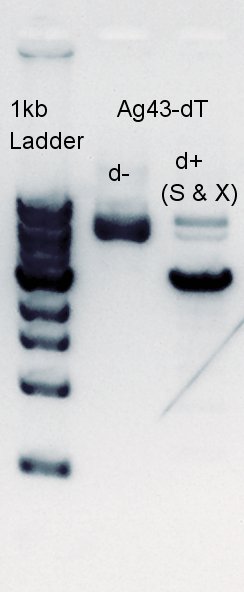
| + | [[image:HokkaidoU2012 120724 ag43-dplus.jpg|thumb|Ag43-dT d+(X&S) Digestion result]] | ||
Electrophoresis for digestion results. | Electrophoresis for digestion results. | ||
| - | + | From this result, we found that '''there are 6 PstI cutting sites in K346007(Ag43)'''. | |
| - | + | ||
| - | + | ====Gel extraction==== | |
| - | ==Gel extraction== | + | Gel extraction of Ag43-dt digestion result. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution. |
| - | + | ||
| - | Gel | + | ====Digestion==== |
| - | </ | + | Ag43-dT and pSB1AK3 mixture solution(each DNA fragment is about 3kbp) digested with HindIII to digest pSB1AK3. |
| - | + | ||
| + | {|class="hokkaidou-table-digestion" | ||
| + | |- | ||
| + | |DNA solution | ||
| + | |8 ul | ||
| + | |- | ||
| + | |HindIII | ||
| + | |1 ul | ||
| + | |- | ||
| + | |10xM buffer | ||
| + | |1 ul | ||
| + | |- | ||
| + | |Total | ||
| + | |10 ul | ||
| + | |} | ||
| + | |||
| + | <br style="line-height: 0; clear: both;" /> | ||
</div></div> | </div></div> | ||
| + | |||
| + | <div class="hokkaidou-notebook-daily"> | ||
| + | ===July 25th=== | ||
| + | <div class="hokkaidou-section"> | ||
| + | ====Digestion==== | ||
| + | Digestion of pT7-RBS on pSB1K3 with SpeI. | ||
| + | |||
| + | {|class="hokkaidou-table-digestion" | ||
| + | |- | ||
| + | |DNA solution | ||
| + | |3 ul | ||
| + | |- | ||
| + | |SpeI | ||
| + | |1 ul | ||
| + | |- | ||
| + | |10xH buffer | ||
| + | |1 ul | ||
| + | |- | ||
| + | |DW | ||
| + | |5 ul | ||
| + | |- | ||
| + | |Total | ||
| + | |10 ul | ||
| + | |} | ||
| + | [[image:HokkaidoU2012 120725 Digestion Ag43-dt on pSB1AK3(HindIII) & pT7-RBS on pSB1K3(SpeI).jpg|thumb|Digestion result(Ag43-dT and pT7-RBS)]] | ||
| + | We were confirmed that pSB1AK3 was digested and became 1.3k and 1.8k bp fragments by HindIII. | ||
| + | |||
| + | ====Gel extraction==== | ||
| + | Gel extraction of Ag43-dT on pSB1AK3(HindIII) and pT7-RBS on pSB1K3(SpeI). | ||
| + | We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution. | ||
| + | |||
| + | ====Ethanol precipitation==== | ||
| + | Ethanol precipitation of digestion and gel extraction products. | ||
| + | #Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. | ||
| + | #Centrifuged at 15000 rpm, 10 min at 4C. | ||
| + | #Removed supernatant and added 220 ul of 70% ethanol. | ||
| + | #Centrifuged at 15000 rpm, 5 min at 4C. | ||
| + | #Removed supernatant and dried out at room temperature after that added 5 ul of DW. | ||
| + | |||
| + | ====Ligation==== | ||
| + | Ligation for pT7-RBS on pSB1K3(Vector) and Ag43-dT(Insert). | ||
| + | {|class="hokkaidou-table-ligation" | ||
| + | |- | ||
| + | |pT7-RBS on pSB1K3 | ||
| + | |2 ul | ||
| + | |- | ||
| + | |Ag43-dT | ||
| + | |2 ul | ||
| + | |- | ||
| + | |DW | ||
| + | |1 ul | ||
| + | |- | ||
| + | |Ligation Mighty Mix(TAKARA) | ||
| + | |5 ul | ||
| + | |- | ||
| + | |Total | ||
| + | |10 ul | ||
| + | |} | ||
| + | |||
| + | |||
| + | {|class="hokkaidou-table-ligation" | ||
| + | |- | ||
| + | |Degree | ||
| + | |Minute | ||
| + | |- | ||
| + | |16 | ||
| + | |30 | ||
| + | |- | ||
| + | |65 | ||
| + | |10 | ||
| + | |- | ||
| + | |4 | ||
| + | |Hold | ||
| + | |} | ||
| + | |||
| + | <br style="line-height: 0; clear: both;" /> | ||
| + | </div></div> | ||
| + | |||
| + | <div class="hokkaidou-notebook-daily"> | ||
| + | ===July 26th=== | ||
| + | <div class="hokkaidou-section"> | ||
| + | ====Transformation==== | ||
| + | Transformation plasmid DNA ligated at 25th (pT7-RBS-Ag43-dT on pSB1K3) into BL21. | ||
| + | #Added 2 ul of plasmid DNA to 50 ul of thawed competent cells on ice. | ||
| + | #Incubated on ice for 30 min. | ||
| + | #Added 200 ul of LB then incubated the cells for 2 hrs at 37C. | ||
| + | #Prepared and Labeled two petri dishes with LBK. | ||
| + | #Spread 200 ul of the transformation onto first dish. | ||
| + | #Added 450 ul of LB to 50 ul of the transformation and spread 200 ul of it onto second dish. | ||
| + | #Incubated the plates at 37C for over 30 hrs. | ||
| + | |||
| + | There were no colony on the plates. | ||
| + | |||
| + | ====Electrophoresis==== | ||
| + | Electrophoresis of digestion and ligation products. | ||
| + | #Placed TBE agarose gel in Electrophoresis chamber. | ||
| + | #Added 1/2X TBE buffer to Electrophoresis chamber. | ||
| + | #Added 5 ul of EtBr and ran at 100 V for 30 min. | ||
| + | #Apply 1kb DNA ladder and each samples. | ||
| + | #Ran at 100 V for 30 min. | ||
| + | [[image:HokkaidoU2012 120726 digestion ligation.jpg|thumb|Digestion and Ligation results]] | ||
| + | There are no band in the lane of ligation products. But if digestion products were not ligated, two bands of digestion products would exist in the lane. | ||
| + | |||
| + | <br style="line-height: 0; clear: both;" /> | ||
| + | </div></div> | ||
| + | |||
| + | <div class="hokkaidou-notebook-daily"> | ||
| + | ===July 27th=== | ||
| + | <div class="hokkaidou-section"> | ||
| + | ====Transformation==== | ||
| + | Transformation plasmid DNA ligated at 25th (pT7-RBS-Ag43-dT on pSB1K3) into BL21. | ||
| + | #Added 2 ul of plasmid DNA to 50 ul of thawed competent cells on ice. | ||
| + | #Incubated on ice for 30 min. | ||
| + | #Added 600 ul of LB then incubated the cells for 2 hrs at 37C. | ||
| + | #Prepared and Labeled three petri dishes with LBK X2 and LBC. | ||
| + | #Spread 300 ul of the transformation onto LBK dish. | ||
| + | #Added 900 ul of LB to 100 ul of the transformation and spread 300 ul of it onto LBC dish and LBK dish. | ||
| + | #Incubated the plates at 37C for 17 hrs and 30 min. | ||
| + | |||
| + | ====Ligation==== | ||
| + | Ligation of pT7-RBS on pSB1K3(Vector) and Ag43-dT(Insert) | ||
| + | {|class="hokkaidou-table-ligation" | ||
| + | |- | ||
| + | |pT7-RBS on pSB1K3 | ||
| + | |2 ul | ||
| + | |- | ||
| + | |Ag43-dT | ||
| + | |2 ul | ||
| + | |- | ||
| + | |DW | ||
| + | |1 ul | ||
| + | |- | ||
| + | |Ligation Mighty Mix(TAKARA) | ||
| + | |5 ul | ||
| + | |- | ||
| + | |Total | ||
| + | |10 ul | ||
| + | |} | ||
| + | |||
| + | |||
| + | {|class="hokkaidou-table-ligation" | ||
| + | |- | ||
| + | |Degree | ||
| + | |Minute | ||
| + | |- | ||
| + | |16 | ||
| + | |30 | ||
| + | |- | ||
| + | |65 | ||
| + | |10 | ||
| + | |- | ||
| + | |4 | ||
| + | |Hold | ||
| + | |} | ||
| + | |||
| + | <br style="line-height: 0; clear: both;" /> | ||
| + | </div></div> | ||
| + | |||
| + | <div class="hokkaidou-notebook-daily"> | ||
| + | ===July 28th=== | ||
| + | <div class="hokkaidou-section"> | ||
| + | [[image:HokkaidoU2012 120728 Transformation result.jpg|thumb|Transformation result left:LBC center:LBK right:LBK]] | ||
| + | There were many colonies on LBC. We guessed we mistook pT7-RBS on pSB1C3 for pT7-RBS on pSB1K3. | ||
| + | |||
| + | ====Liquid culture==== | ||
| + | Liquid culture for pT7-RBS-Ag43-dT on pSB1C3. | ||
| + | #Added 2 ml of LBC into culture tubes. | ||
| + | #Resuspended 5 colonies. | ||
| + | #Incubated the tubes at 30C for 22 hrs. | ||
| + | |||
| + | ====Digestion==== | ||
| + | Digestion of pT7-RBS on pSB1K3 with SpeI and Ag43-dT on pSB1AK3(cut with SpeI & XbaI) with HindIII. | ||
| + | <br /> | ||
| + | pT7-RBS on pSB1K3 | ||
| + | {|class="hokkaidou-table-digestion" | ||
| + | |- | ||
| + | |DNA solution | ||
| + | |3 ul | ||
| + | |- | ||
| + | |SpeI | ||
| + | |1 ul | ||
| + | |- | ||
| + | |10xH buffer | ||
| + | |1 ul | ||
| + | |- | ||
| + | |DW | ||
| + | |5 ul | ||
| + | |- | ||
| + | |Total | ||
| + | |10 ul | ||
| + | |} | ||
| + | |||
| + | Ag43-dT on pSB1AK3(cut with SpeI & XbaI) | ||
| + | {|class="hokkaidou-table-digestion" | ||
| + | |- | ||
| + | |DNA solution | ||
| + | |8 ul | ||
| + | |- | ||
| + | |HindIII | ||
| + | |1 ul | ||
| + | |- | ||
| + | |10xM buffer | ||
| + | |1 ul | ||
| + | |- | ||
| + | |Total | ||
| + | |10 ul | ||
| + | |} | ||
| + | |||
| + | Digested at 37C for 2 hrs. | ||
| + | [[image:HokkaidoU2012 120728 Digestion pT7-RBS on pSB1K3(SpeI) Ag43-dT on pSB1AK3(HindIII).jpg|thumb|digestion result]] | ||
| + | This results showed that pSB1AK3 was successfully digested into fragments(1.3K and 1.8K bp), but we couldn't confirm whether pT7-RBS on pSB1K3 was digested or not. | ||
| + | |||
| + | ====Gel extraction==== | ||
| + | Gel extraction for digestion production. We used FastGene Gel&PCR extraction kit(NipponGenetics) and got 50 ul of DNA solution. | ||
| + | |||
| + | ====Ethanol precipitation==== | ||
| + | Ethanol precipitation for digestion and gel extraction product. | ||
| + | #Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. | ||
| + | #Centrifuged at 15000 rpm, 10 min at 4C. | ||
| + | #Removed supernatant and added 220 ul of 70% ethanol. | ||
| + | #Centrifuged at 15000 rpm, 10 min at 4C. | ||
| + | #Removed supernatant and dryed out at room temperature after that added 10 ul of DW. | ||
| + | |||
| + | ====Ligation==== | ||
| + | Ligation of pT7-RBS on pSB1K3(Vector) and Ag43-dT(Insert) | ||
| + | |||
| + | {|class="hokkaidou-table-ligation" | ||
| + | |- | ||
| + | |pT7-RBS on pSB1K3 | ||
| + | |2 ul | ||
| + | |- | ||
| + | |Ag43-dT | ||
| + | |2 ul | ||
| + | |- | ||
| + | |DW | ||
| + | |1 ul | ||
| + | |- | ||
| + | |Ligation Mighty Mix(TAKARA) | ||
| + | |5 ul | ||
| + | |- | ||
| + | |Total | ||
| + | |10 ul | ||
| + | |} | ||
| + | |||
| + | |||
| + | {|class="hokkaidou-table-ligation" | ||
| + | |- | ||
| + | |Degree | ||
| + | |Minute | ||
| + | |- | ||
| + | |16 | ||
| + | |30 | ||
| + | |- | ||
| + | |65 | ||
| + | |10 | ||
| + | |- | ||
| + | |4 | ||
| + | |Hold | ||
| + | |} | ||
| + | |||
| + | <br style="line-height: 0; clear: both;" /> | ||
| + | </div></div> | ||
| + | |||
| + | <div class="hokkaidou-notebook-daily"> | ||
| + | ===July 29th=== | ||
| + | <div class="hokkaidou-section"> | ||
| + | ====Plasmid extraction==== | ||
| + | Plasmid extraction for 5 cultures of pT7-RBS-Ag43-dT. We used FastGene Plasmid Mini Kit(Nippon Genetics) and got 50 ul of DNA solutions. | ||
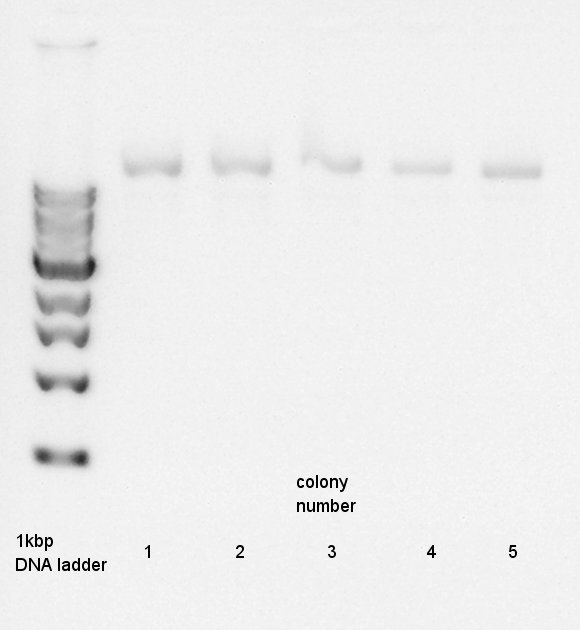
| + | [[image:HokkaidoU2012_120729_pt7-rbs-ag43-dt.jpg|thumb|Plasmid extraction products]] | ||
| + | |||
| + | ====Digestion==== | ||
| + | We need to confirm the DNA is pT7-RBS-Ag43-dT or not.<br /> | ||
| + | Plasmid extraction products(pT7-RBS-Ag43-dT) | ||
| + | PstI | ||
| + | {|class="hokkaidou-table-digestion" | ||
| + | |- | ||
| + | |DNA solution | ||
| + | |4 ul | ||
| + | |- | ||
| + | |PstI | ||
| + | |1 ul | ||
| + | |- | ||
| + | |10xH buffer | ||
| + | |2 ul | ||
| + | |- | ||
| + | |DW | ||
| + | |13 ul | ||
| + | |- | ||
| + | |Total | ||
| + | |20 ul | ||
| + | |} | ||
| + | |||
| + | |||
| + | Plasmid extraction products(pT7-RBS-Ag43-dT)<br /> | ||
| + | EcoR1 and Spe1 | ||
| + | {|class="hokkaidou-table-digestion" | ||
| + | |- | ||
| + | |DNA solution | ||
| + | |4 ul | ||
| + | |- | ||
| + | |EcoR1 | ||
| + | |1 ul | ||
| + | |- | ||
| + | |Spe1 | ||
| + | |1 ul | ||
| + | |- | ||
| + | |10xH buffer | ||
| + | |2 ul | ||
| + | |- | ||
| + | |DW | ||
| + | |12 ul | ||
| + | |- | ||
| + | |Total | ||
| + | |20 ul | ||
| + | |} | ||
| + | |||
| + | ====Electrophoresis==== | ||
| + | Electrophoresis for digestion results. | ||
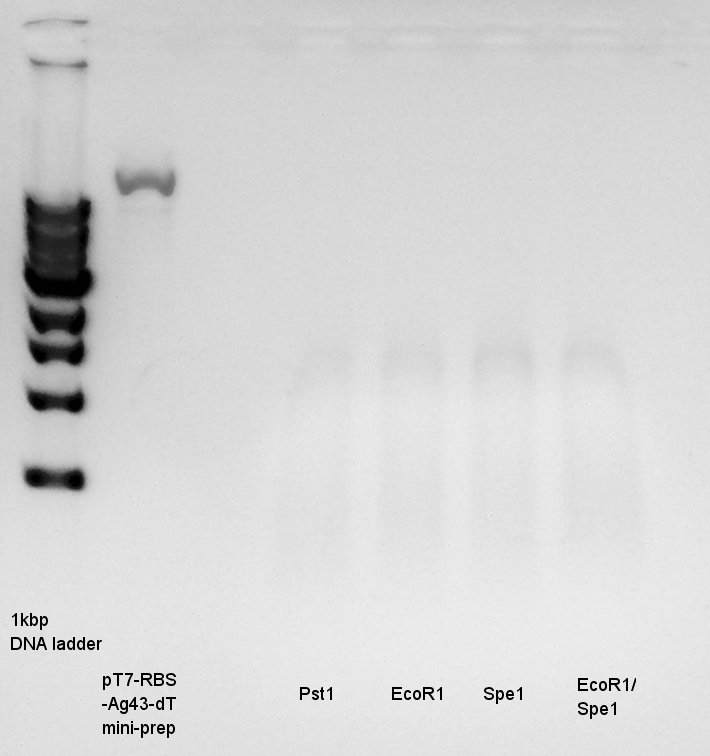
| + | [[image:HokkaidoU2012_120729_ag43-dig.jpg|thumb|Digestion result]] | ||
| + | I could not understand what is happend. | ||
| + | I tried it again, but the result was the same. | ||
| + | |||
| + | <br style="line-height: 0; clear: both;" /> | ||
| + | </div></div> | ||
| + | |||
<!-- DO NOT EDIT UNDER THIS LINE @iTakeshi --> | <!-- DO NOT EDIT UNDER THIS LINE @iTakeshi --> | ||
</div> | </div> | ||
<br style="line-height: 0; clear: both;" /> | <br style="line-height: 0; clear: both;" /> | ||
{{Team:HokkaidoU_Japan/footer}} | {{Team:HokkaidoU_Japan/footer}} | ||
Latest revision as of 03:18, 27 September 2012
Contents |
July 23rd
Plasmid extraction
Plasmid extraction for Ag43(resuspended colony incubated at July 17th and resuspended colony incubated at July 20th). We used FastGene Plasmid Mini Kit(Nippon Genetics) and got 50 ul of DNA solutions.
July 24th
Electrophoresis
Electrophoresis for Ag43(plasmid extraction at July 23rd) and Ag43 digestion results(digested with EcoRI and SpeI)
From this digestion result, we found out that one or two enzymes didn't work successfully but there are enough concentration of DNA in 3000bp band to use for digestion.
Gel extraction
Gel extraction for digestion production. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution.
Ethanol precipitation
Ethanol precipitation for digestion and gel extraction product.
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged at 15000 rpm, 10min at 4C.
- Removed supernatant and added 220 ul of 70% ethanol.
- Centrifuged at 15000 rpm, 10 min at 4C.
- Removed supernatant and dried out at room temperature after that added 10 ul of DW.
From this result, we estimated that the concentration of ethanol precipitation product is about 40 ng/ul.
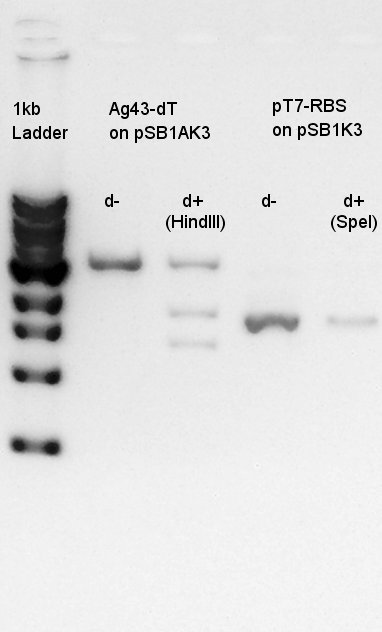
Digestion
Digestion to confirm how many PstI cutting sites are there in K346007 and for Ag43-dT complex with SpeI and XbaI.
Ag43
PstI
| DNA solution | 5 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 12 ul |
| Total | 20 ul |
Ag43-dT
SpeI and XbaI
| DNA solution | 12 ul |
| SpeI | 1 ul |
| XbaI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 4 ul |
| Total | 20 ul |
Electrophoresis
Electrophoresis for digestion results.
From this result, we found that there are 6 PstI cutting sites in K346007(Ag43).
Gel extraction
Gel extraction of Ag43-dt digestion result. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution.
Digestion
Ag43-dT and pSB1AK3 mixture solution(each DNA fragment is about 3kbp) digested with HindIII to digest pSB1AK3.
| DNA solution | 8 ul |
| HindIII | 1 ul |
| 10xM buffer | 1 ul |
| Total | 10 ul |
July 25th
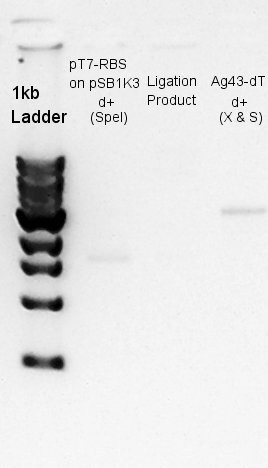
Digestion
Digestion of pT7-RBS on pSB1K3 with SpeI.
| DNA solution | 3 ul |
| SpeI | 1 ul |
| 10xH buffer | 1 ul |
| DW | 5 ul |
| Total | 10 ul |
We were confirmed that pSB1AK3 was digested and became 1.3k and 1.8k bp fragments by HindIII.
Gel extraction
Gel extraction of Ag43-dT on pSB1AK3(HindIII) and pT7-RBS on pSB1K3(SpeI). We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution.
Ethanol precipitation
Ethanol precipitation of digestion and gel extraction products.
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged at 15000 rpm, 10 min at 4C.
- Removed supernatant and added 220 ul of 70% ethanol.
- Centrifuged at 15000 rpm, 5 min at 4C.
- Removed supernatant and dried out at room temperature after that added 5 ul of DW.
Ligation
Ligation for pT7-RBS on pSB1K3(Vector) and Ag43-dT(Insert).
| pT7-RBS on pSB1K3 | 2 ul |
| Ag43-dT | 2 ul |
| DW | 1 ul |
| Ligation Mighty Mix(TAKARA) | 5 ul |
| Total | 10 ul |
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
July 26th
Transformation
Transformation plasmid DNA ligated at 25th (pT7-RBS-Ag43-dT on pSB1K3) into BL21.
- Added 2 ul of plasmid DNA to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30 min.
- Added 200 ul of LB then incubated the cells for 2 hrs at 37C.
- Prepared and Labeled two petri dishes with LBK.
- Spread 200 ul of the transformation onto first dish.
- Added 450 ul of LB to 50 ul of the transformation and spread 200 ul of it onto second dish.
- Incubated the plates at 37C for over 30 hrs.
There were no colony on the plates.
Electrophoresis
Electrophoresis of digestion and ligation products.
- Placed TBE agarose gel in Electrophoresis chamber.
- Added 1/2X TBE buffer to Electrophoresis chamber.
- Added 5 ul of EtBr and ran at 100 V for 30 min.
- Apply 1kb DNA ladder and each samples.
- Ran at 100 V for 30 min.
There are no band in the lane of ligation products. But if digestion products were not ligated, two bands of digestion products would exist in the lane.
July 27th
Transformation
Transformation plasmid DNA ligated at 25th (pT7-RBS-Ag43-dT on pSB1K3) into BL21.
- Added 2 ul of plasmid DNA to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30 min.
- Added 600 ul of LB then incubated the cells for 2 hrs at 37C.
- Prepared and Labeled three petri dishes with LBK X2 and LBC.
- Spread 300 ul of the transformation onto LBK dish.
- Added 900 ul of LB to 100 ul of the transformation and spread 300 ul of it onto LBC dish and LBK dish.
- Incubated the plates at 37C for 17 hrs and 30 min.
Ligation
Ligation of pT7-RBS on pSB1K3(Vector) and Ag43-dT(Insert)
| pT7-RBS on pSB1K3 | 2 ul |
| Ag43-dT | 2 ul |
| DW | 1 ul |
| Ligation Mighty Mix(TAKARA) | 5 ul |
| Total | 10 ul |
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
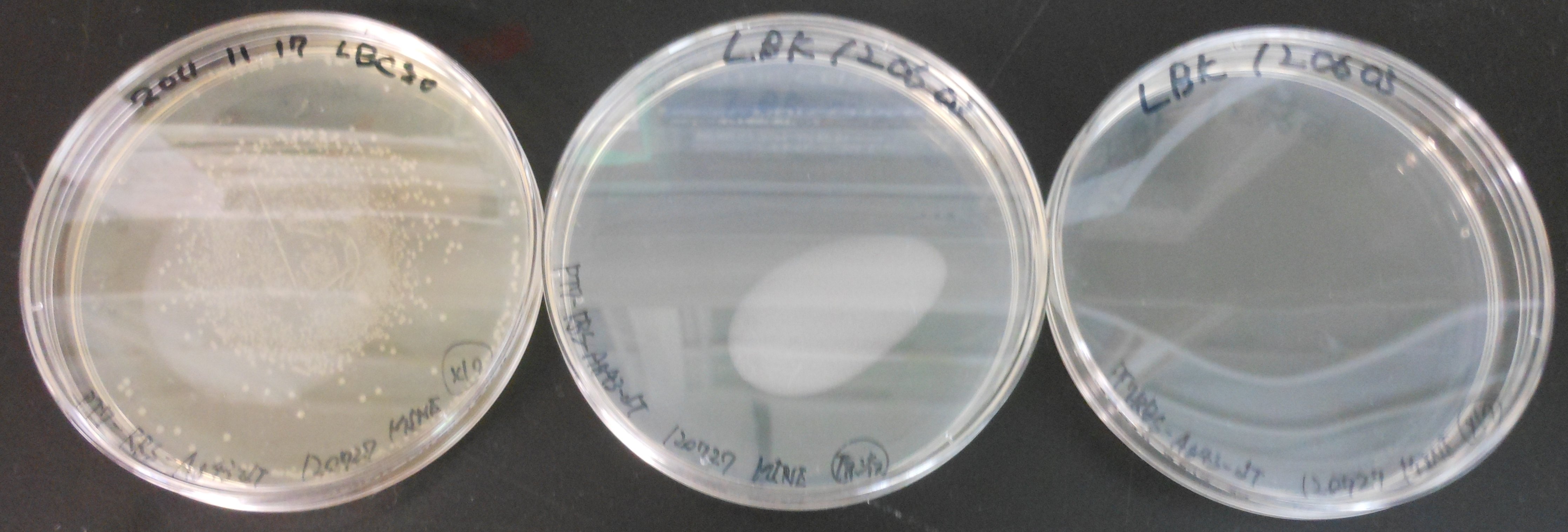
July 28th
There were many colonies on LBC. We guessed we mistook pT7-RBS on pSB1C3 for pT7-RBS on pSB1K3.
Liquid culture
Liquid culture for pT7-RBS-Ag43-dT on pSB1C3.
- Added 2 ml of LBC into culture tubes.
- Resuspended 5 colonies.
- Incubated the tubes at 30C for 22 hrs.
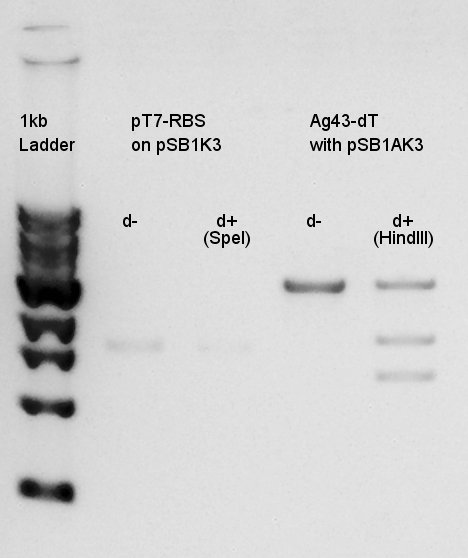
Digestion
Digestion of pT7-RBS on pSB1K3 with SpeI and Ag43-dT on pSB1AK3(cut with SpeI & XbaI) with HindIII.
pT7-RBS on pSB1K3
| DNA solution | 3 ul |
| SpeI | 1 ul |
| 10xH buffer | 1 ul |
| DW | 5 ul |
| Total | 10 ul |
Ag43-dT on pSB1AK3(cut with SpeI & XbaI)
| DNA solution | 8 ul |
| HindIII | 1 ul |
| 10xM buffer | 1 ul |
| Total | 10 ul |
Digested at 37C for 2 hrs.
This results showed that pSB1AK3 was successfully digested into fragments(1.3K and 1.8K bp), but we couldn't confirm whether pT7-RBS on pSB1K3 was digested or not.
Gel extraction
Gel extraction for digestion production. We used FastGene Gel&PCR extraction kit(NipponGenetics) and got 50 ul of DNA solution.
Ethanol precipitation
Ethanol precipitation for digestion and gel extraction product.
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged at 15000 rpm, 10 min at 4C.
- Removed supernatant and added 220 ul of 70% ethanol.
- Centrifuged at 15000 rpm, 10 min at 4C.
- Removed supernatant and dryed out at room temperature after that added 10 ul of DW.
Ligation
Ligation of pT7-RBS on pSB1K3(Vector) and Ag43-dT(Insert)
| pT7-RBS on pSB1K3 | 2 ul |
| Ag43-dT | 2 ul |
| DW | 1 ul |
| Ligation Mighty Mix(TAKARA) | 5 ul |
| Total | 10 ul |
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
July 29th
Plasmid extraction
Plasmid extraction for 5 cultures of pT7-RBS-Ag43-dT. We used FastGene Plasmid Mini Kit(Nippon Genetics) and got 50 ul of DNA solutions.
Digestion
We need to confirm the DNA is pT7-RBS-Ag43-dT or not.
Plasmid extraction products(pT7-RBS-Ag43-dT)
PstI
| DNA solution | 4 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 13 ul |
| Total | 20 ul |
Plasmid extraction products(pT7-RBS-Ag43-dT)
EcoR1 and Spe1
| DNA solution | 4 ul |
| EcoR1 | 1 ul |
| Spe1 | 1 ul |
| 10xH buffer | 2 ul |
| DW | 12 ul |
| Total | 20 ul |
Electrophoresis
Electrophoresis for digestion results.
I could not understand what is happend. I tried it again, but the result was the same.
 "
"