Team:HokkaidoU Japan/Notebook/aggregation Week 6
From 2012.igem.org
Contents |
August 6th
Ethanol precipitation
Ethanol precipitation for digestion and gel extraction product.
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged in 15000 rpm, 10 min at 4C.
- Remove supernatant and added 220 ul of 70% ethanol.
- Centrifuged in 15000 rpm, 10 min at 4C.
- Remove supernatant and air drying in room temperature then added 10 ul of DW.
Ligation
We ligated pT7-RBS on pSB1K3 (digested Spe1) as vector and Ag43-dT (digested Spe1 and Xba1 and HindIII) as insert.
| pT7-RBS | 1 ul |
| Ag43-dT | 2 ul |
| DW | 2 ul |
| Ligation Mighty Mix(TAKARA) | 5 ul |
| Total | 10 ul |
Ligation reaction time was written below.
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
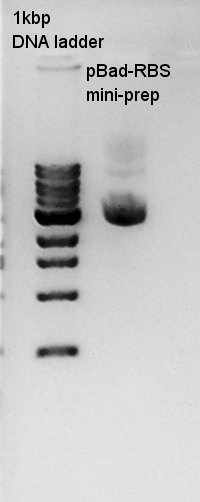
mini-prep
mini-prep of pBad-RBS. We used FastGene Plasmid Mini Kit(Nippon Genetics) and got 50 ul of DNA solutions.
transformation
Transformation of plasmid DNA (pT7-RBS-Ag43-dT on pSB1K3)
in E. coli(DH5α).
- Added 2 ul of plasmid DNA to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30 min.
- Added 600 ul of LB then incubated the cells for 2 hours at 37C.
- Prepared and Labeled two LBK plates.
- Plated 300 ul of the culture onto first dish and spread.
- Added 900 ul of LB to 100 ul of the culture and plated 300 ul of it onto second dish and spread.
- Incubated them at 37C for hours.
 "
"