Team:Bielefeld-Germany/Results/halo
From 2012.igem.org

Summary
First some trials of shaking flask cultivations were made with various parameters to identify the best conditions for production of the His tagged laccase Lbh1 from [http://www.dsmz.de/catalogues/details/culture/DSM-18197.html?tx_dsmzresources_pi5 Bacillus halodurans C-125 ] named BHAL. Due to inactivity of the enzyme in the cell lysate a purification method was established (using Ni-NTA-Histag resin). BHAL could not be detected by SDS-PAGE (theoretical molecular weight of 56 kDa) or activity test by using the BioBrick <partinfo>BBa_K863020</partinfo> and E. coli KRX as expression system. Due to this results the new BioBrick <partinfo>BBa_K863022</partinfo> was constructed and expressed E. coli Rossetta-Gami 2. With this expression system the laccase could be produced and analysed via SDS-PAGE. A small scale Ni-NTA-column was used to purify the laccase. The fractionated samples were tested regarding their activity with ABTS and showed ability in oxidizing ABTS. A scale up to 12 L with a optimized medium (HSG) and a labscale Ni-NTA-Purification were implemented to enable additional experiments to characterize BHAL.
Cultivation, Purification and SDS-PAGE
Cultivation
The first trials to produce the Lbh1 - laccase from Bacillus halodurans (named BHAL) were performed in shaking flasks with various flask designs (from 100 mL-1 to 1 L flasks, with and without baffles) and under several conditions. The varied parameters in our screening experiments were temperature (27 °C,30 °C and 37 °C), concentration of chloramphenicol (20-170 µg mL-1), induction strategy (autoinduction and manual induction with 0,1 % rhamnose) and cultivation time (6 to 24 h). Furthermore we cultivated with and without 0.25 mM CuCl2 to provide a sufficient amount of copper, which is needed for the active center of the laccase. E.coli KRX was not able to produce active BHAL under the tested conditions, therefore another chassis was chosen. For further cultivations E. coli Rosetta-Gami 2 was transformed with BBa_K863012, because of its ability to translate rare codons. BHAL was produced under the following conditions:
- flask design: shaking flask without baffles
- medium: LB-Medium
- antibiotics: 60 µg mL-1 chloramphenicol and 300 µg mL-1 ampicillin
- temperature: 37 °C
- cultivation time: 24 h
Purification
The cells were harvested and resuspended in Ni-NTA-equilibration buffer, mechanically lysed by sonification and centrifuged. After preparing the cell paste the BHALlaccase could not be purified with the 15 mL column, because of the column was not available. For this reason a small scale purification (6 mL) of the supernatant of the lysate was performed with a 1 mL Ni-NTA-column. The elution was collected in 1 mL fractions.
SDS-PAGE
In figure 1 the different fractions of the purified cell lysate of E. coli Rosetta-Gami 2 with <partinfo>BBa_K863022</partinfo> are shown in a SDS-PAGE. BHAL has a molecular weight of 56 kDa. In lane 5, which corresponds to the elution fraction 2, a faint band of 56 kDa is visible. Therefore the fractions were further analysed by activity test and MALDI-TOF.
Since Regionals: 12L Fermentation of E. coli Rosetta-Gami 2 with <partinfo>BBa_K863022</partinfo>
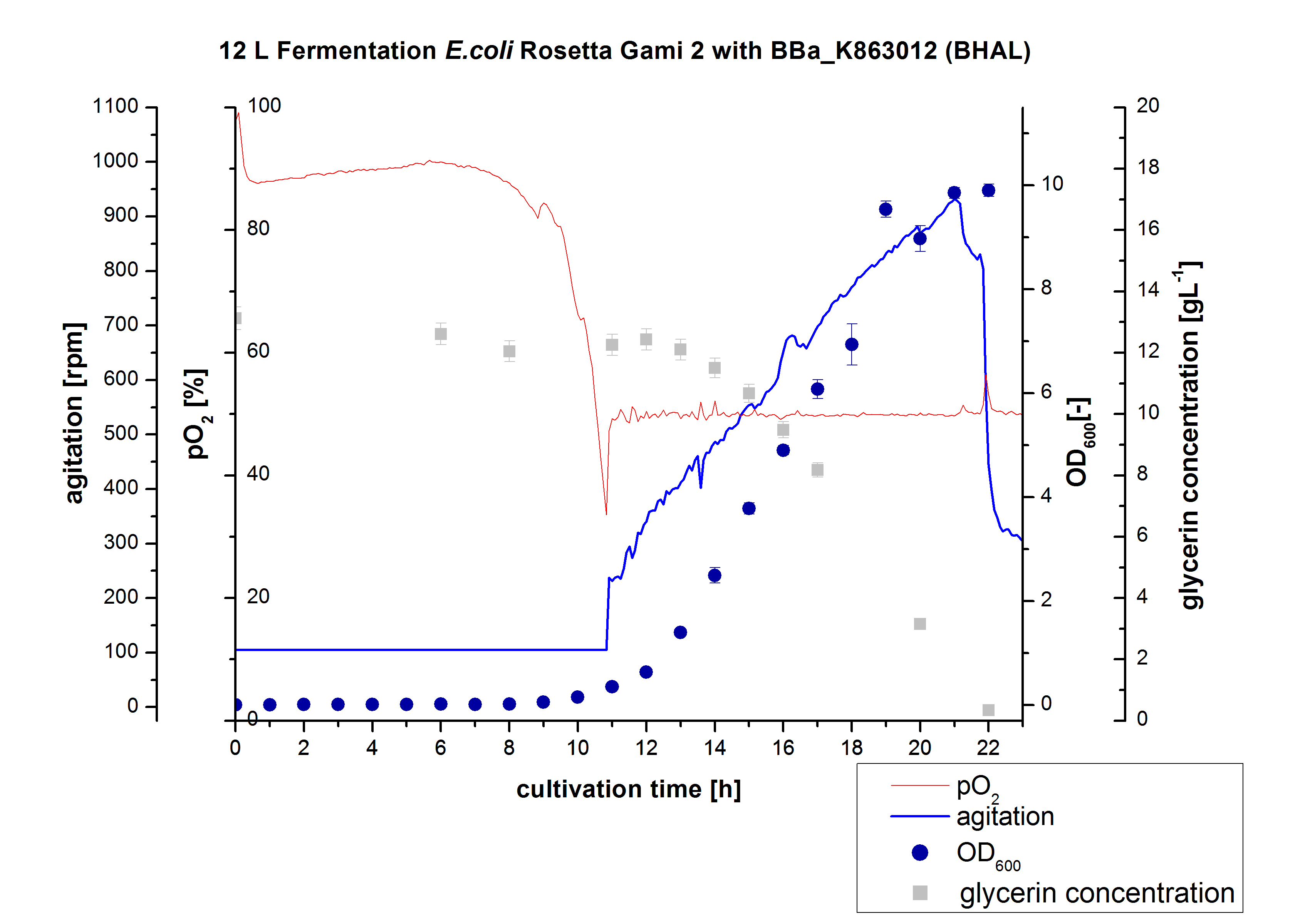
After the measurement of BHAL activity we made a scale-up and fermented E. coli Rosetta-Gami 2 with <partinfo>BBa_K863022</partinfo> in a Bioengineering NFL 22 fermenter with a total volume of 12 L. Agitation speed, pO2 and OD600 were determined and illustrated in Figure 2. This time HSG autodinduction medium was used to produce more biomass. Due to the change of media and to a low amount of cells for inocculation we got a long lag phase of nearly 10 hours. The cell growth caused a decrease in pO2 and after 11 hours the value fell below 50 %, so that the agitation speed increased automatically. After 21 hours the deceleration phase started and therefore the agitation speed was decreased. The maximal OD600 of 9.9 was reached after 22 hours, when the cells were entering the stationary phase. The cells were harvested at this time. It might have been better to cultivate a few hours longer.
Since Regionals: Purification of BHAL
Activity Analysis of [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863022 BHAL]
Initial activity tests of purified fractions
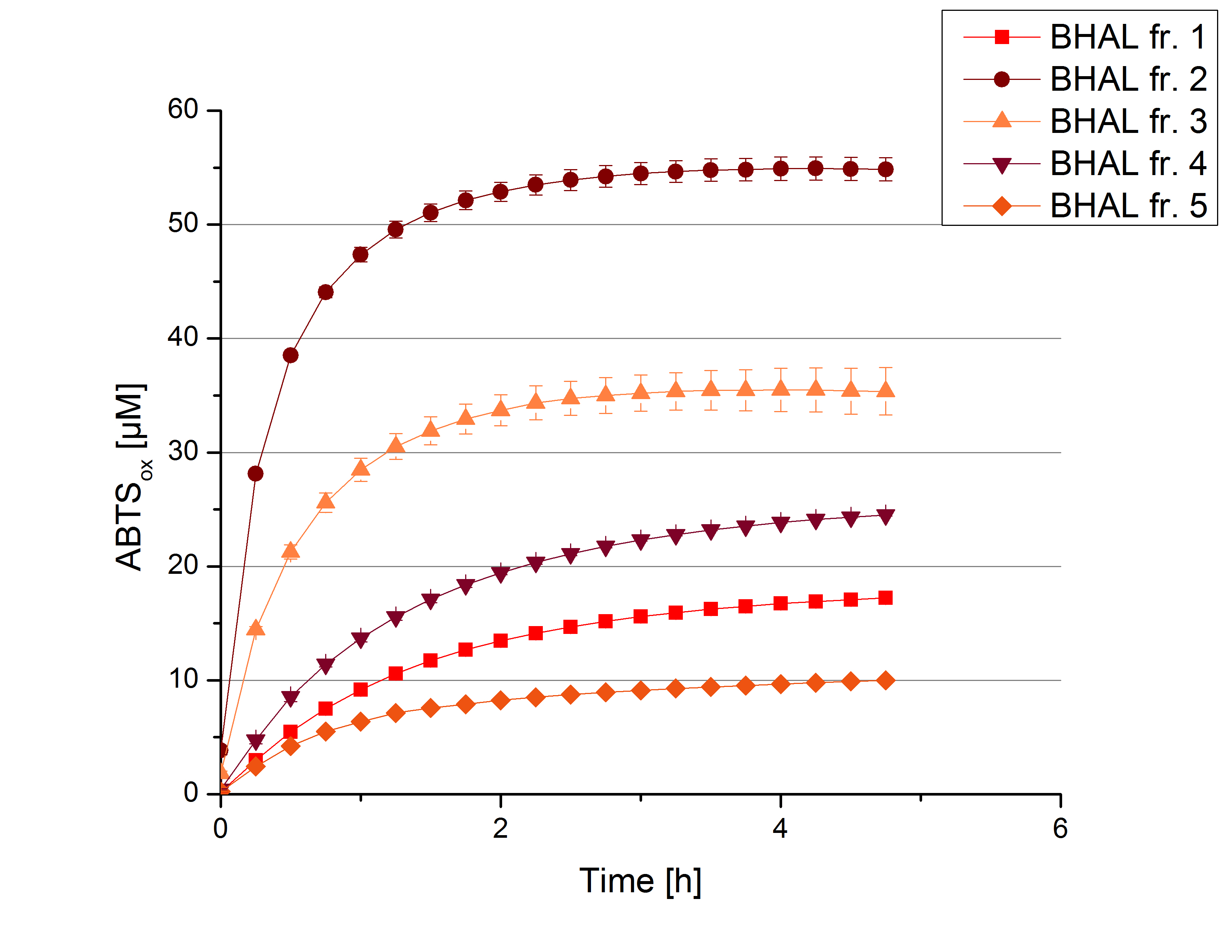
The resulting fractions of the cultivation and purification of [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863022 BHAL] (fraction 1 to 5) were analysed with activity tests. After rebuffering into deionized H2O and incubation with 0.4 mM CuCl2 for 2 hours, the samples were measured with 140 µL sample, 0.1 mM ABTS, 100 mM sodium acetate buffer to a final volume of 200 µL. The change in optical density was measured at 420 nm, reporting the oxidation of ABTS for 5 hours at 25°C. An increase in ABTSox can be seen (Figure 2), indicating produced [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863022 BHAL] laccase in each fraction. Fraction 2 shows the highest amount of ABTSox (55%) reaching saturation after 3 hours. Similar to [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863000 BPUL] laccase, [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863022 BHAL] is capable to reach saturation after 3 hours with approximately oxidizing 55% of the supplied ABTS. Therefore [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863022 BHAL] is going to be characterized further.
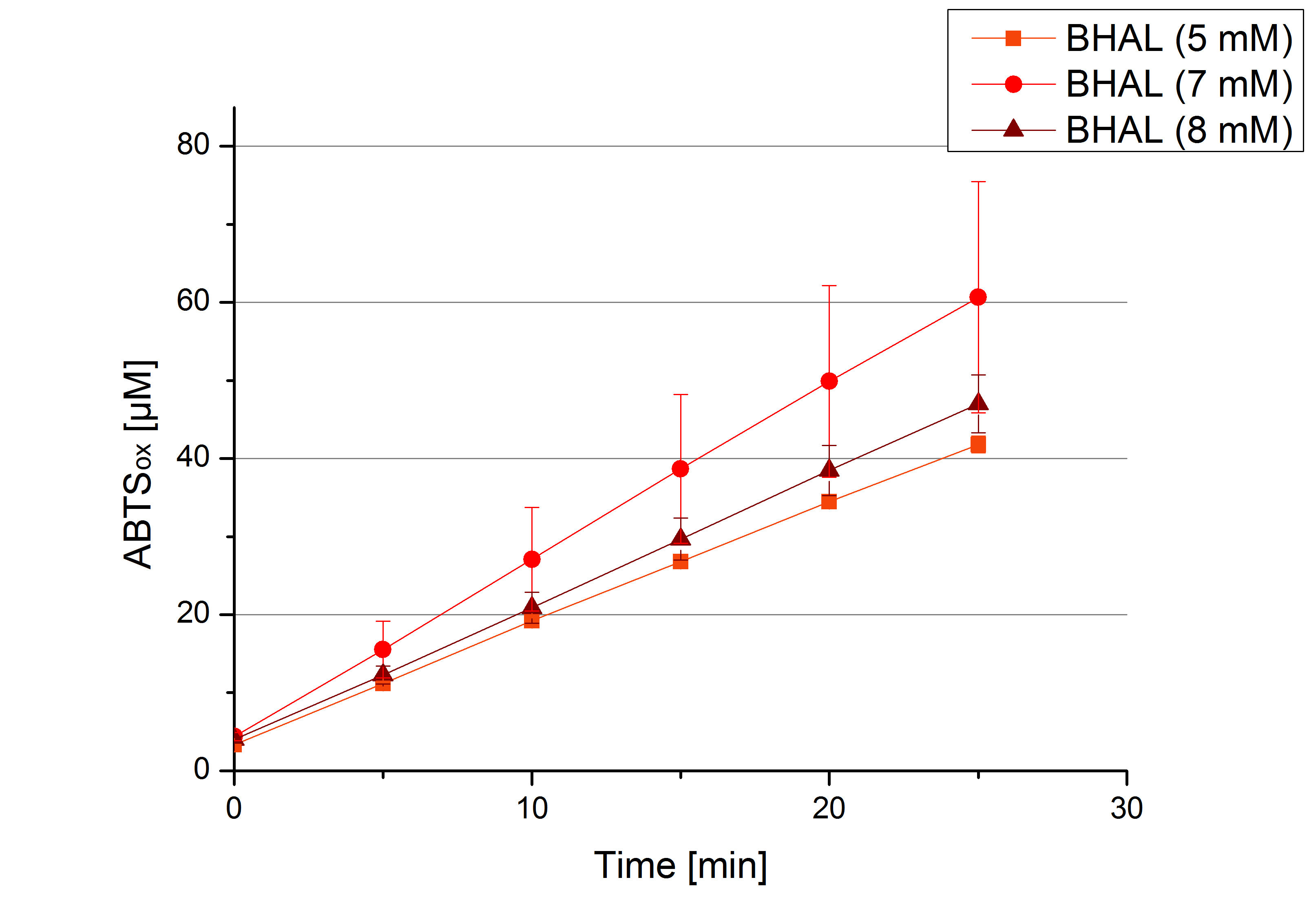
To determine the substrate saturation with ABTS as a substrate, different concentrations of ABTS were used for activity measurements. 616 ng of BHAL laccase were used for measurements in Britton-Robinson buffer (pH 5) and ABTS concentrations ranging from 0.1 mM to 5 mM ABTS, 308 ng of BHAL laccase for measurements ranging from 5 mM to 8 mM.
Since Regionals: Initial activity tests of purified fractions
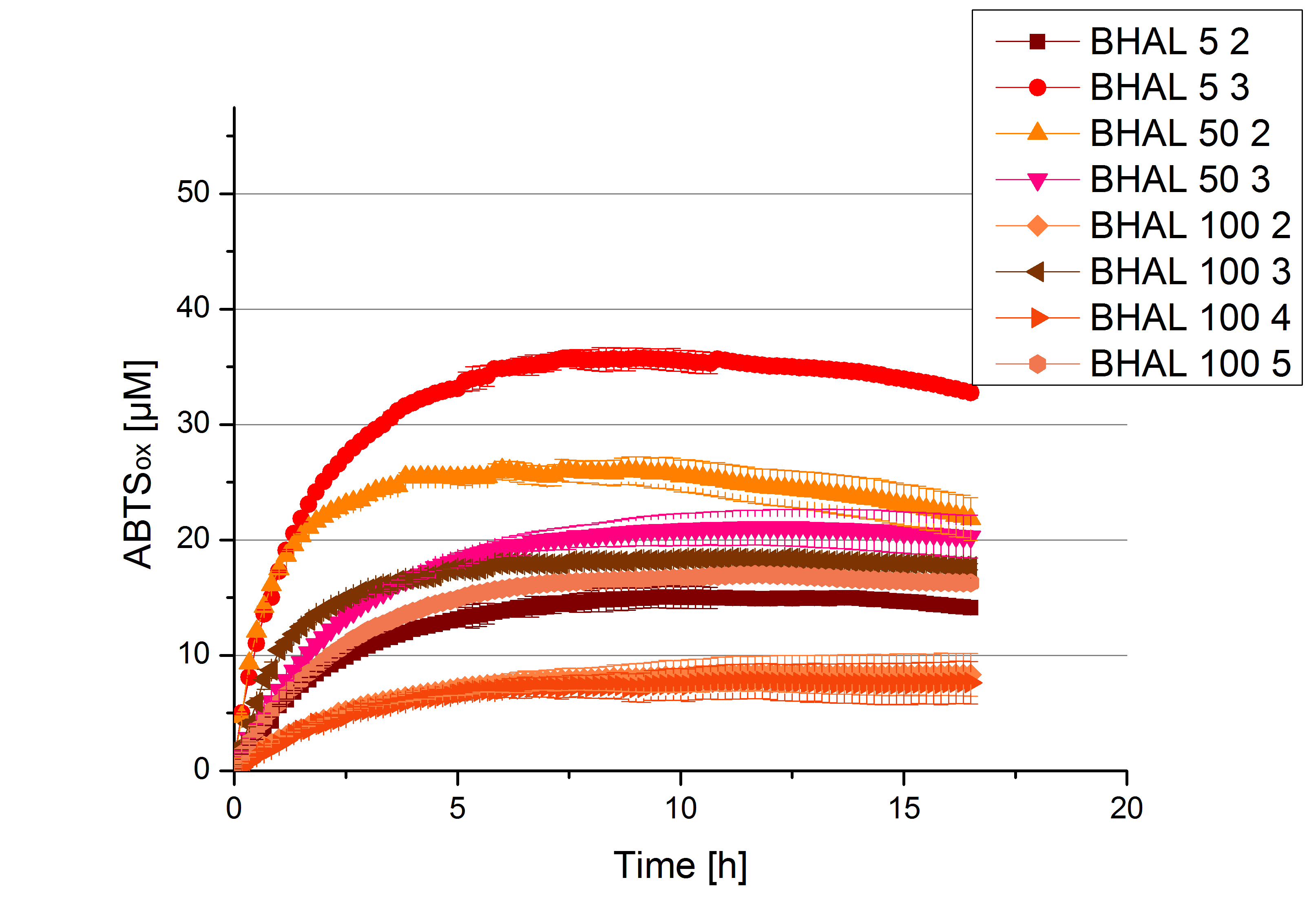
Different fractions of the purification of a new cultivation since the Regional Jamborees in Amsterdam were tested regarding their activity of the produced BHAL enzyme. Before re-buffering, the protein concentration was determined and again after re-buffering. The initial activity tests were done in Britton-Robinson buffer (pH 5) with 0.1 mM ABTS at 25 °C. The protein amount was adjusted in each sample for comparison. One distinct fraction showed the highest activity: fraction 5% 3 (Fig. 3). The contained laccase amount was calculated by assuming, that the most active fraction contains 90 % laccase. This leads to a BHAL laccase concentration 10,9 ng mL-1.
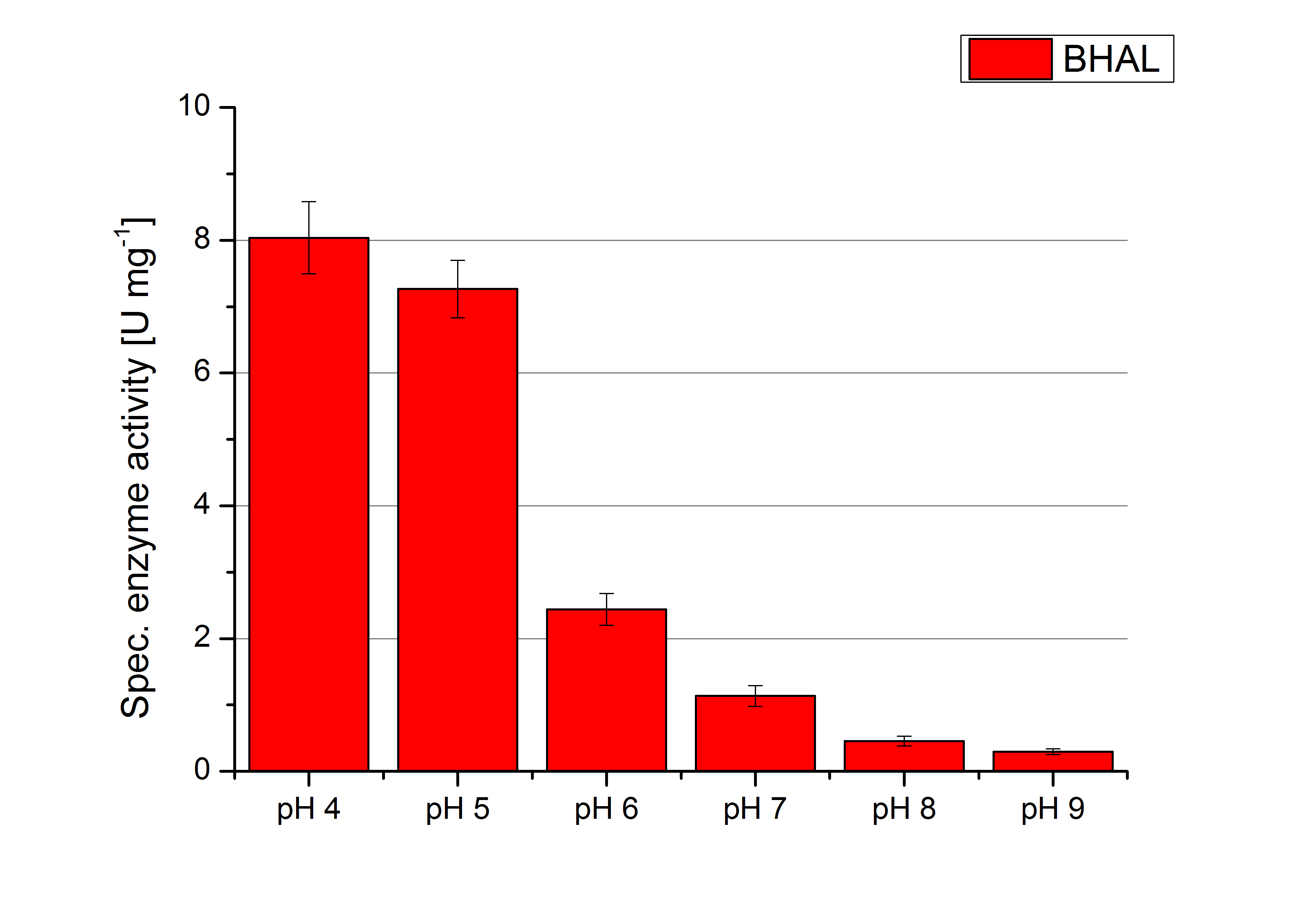
Since Regionals: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863022 BHAL] pH optimum
To determine the optimal experimental setup for BHAL activity measurements the best pH has to be determined. Using Britton-Robinson buffer pHs between pH 4 and pH 9 had been adjusted. 308 ng BHAL laccase per well had been tested under these pH conditions using 7 mM ABTS. The CuCl2 incubated and therefor activated BHAL laccases showed a high activity at pH 4 and pH 5, where most of ABTS has been oxidized (compare to Fig. x and y). The calculated specific enzyme activity of BHAL shows the high activity at both mentioned pHs (Fig. x). While BHAL has an activity of ~8 U mg-1 at pH 4 and pH 5, the enzyme activity decreases at higher pHs. At a pH of 6 only 1/3 of enzyme activity can be detected compared to the activity at pH 4 and pH 5. While still active at pH 7, the BHAL laccase is not as suitable as thought for an application at a waste water treatment plant because of its high activity in acidic environments.

Since Regionals: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863022 BHAL] activity at different temperatures
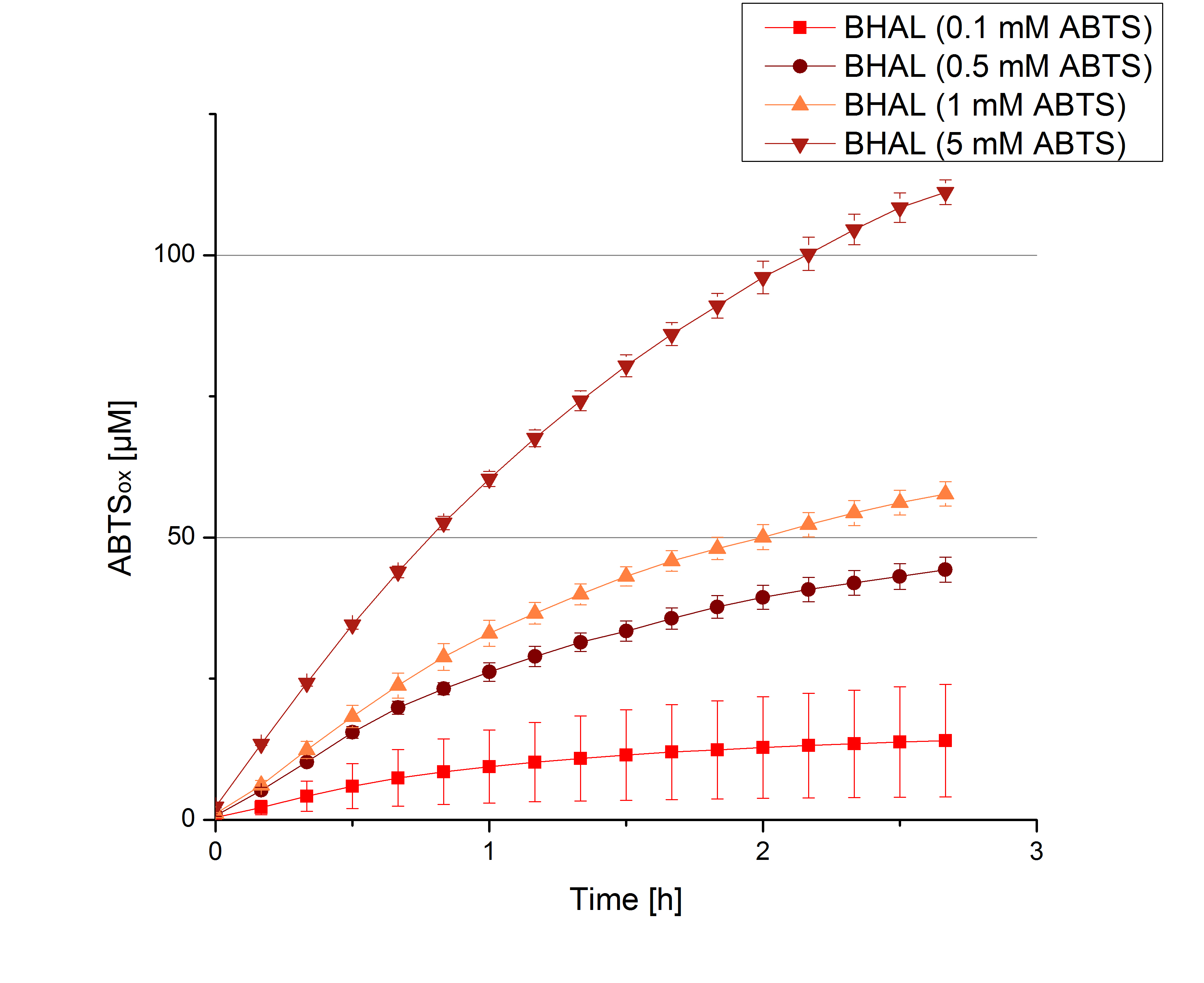
Since Regionals: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863022 BHAL] activity depending on different ABTS concentrations
To be able to calculate the activity in Units mg-1, measurments have to be dne under substrate saturation. This allows the comparison of Units mg-1 with other laccase activities and the literature. For this purpose ABTS concentrations ranging from 0.1 mM to 8 mM were applied in an experimental setup contain Britton-Robinson buffer (pH) and tmeperature conditions of 25 °C. For measurements with 0.1 mM to 5 mM ABTS 616 ng BHAL laccase were used (Fig. x). Measurements with 5 mM to 8 mM ABTS only 308 ng BHAL laccase were applied (Fig. y). Applying less then 7 mM ABTS a static increase in oxidized ABTS is given (compare with Figure x and y). Measurements with 8 mM ABTS show a slower increase in oxidized ABTS as with 7 mM ABTS (Fig. y). This may be due to a substrate toxication. The most compromising ABTS concentration is 7 mM with the highest increase in oxidized ABTS. Therefor a substrate saturation is reached with 7 mM ABTS.
| 55px | | | | | | | | | | |
/div> /p>
 "
"