Team:Bielefeld-Germany/Results/substrate
From 2012.igem.org

Contents |
Introduction
Degradation measurements with high performance liquid chromatography
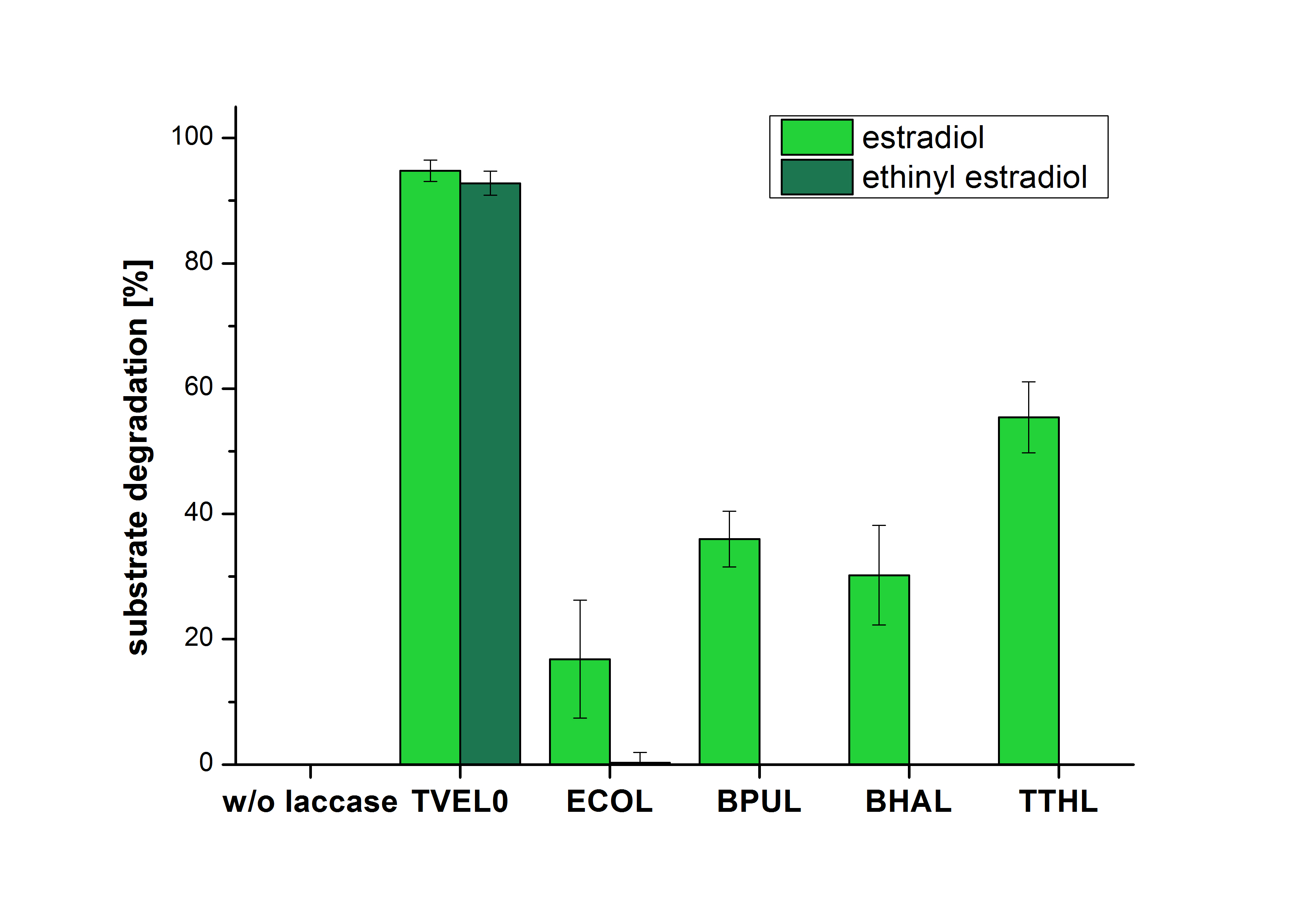
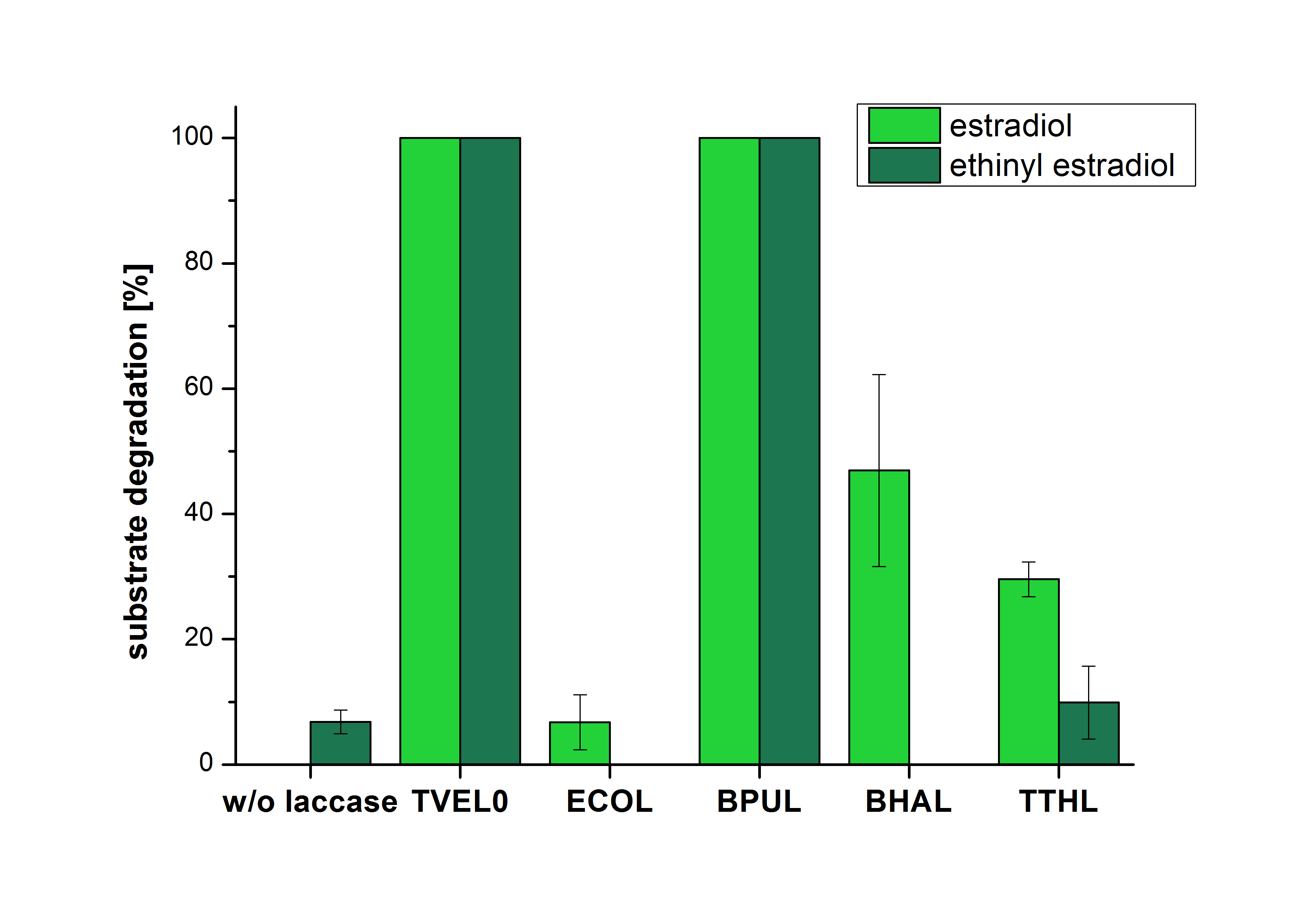
The measurements were made to test if the produced laccases were able to degrade different hormones. Therefore the produced laccases were inserted in the same concentrations (3 µg mL-1) to the different measurement approaches. For the correct pH value (which were measured by the Team Activity Test) Britton Robinson buffer at pH 5 was used for all measurements. The initial substrate concentration was 5 µg mL-1. The results of the reactions without ABTS are shown in Figure 1. On the Y-axis the percentages of degraded estradiol (blue) and ethinyl estradiol (red) are indicated. The X-axis displays the different tested laccases. The degradation was measured at t0 and after five hours of incubation at 30 °C. The negative control was the substrate in Britton Robinson buffer and showed no degradation of the substrates. The bought laccase TVEL0 which is used as positive control is able to degrade 94.7 % estradiol and 92.7 % ethinyl estradiol. The laccase BPUL (from Bacillus pumilus) degraded 35.9 % of used estradiol after five hours. ECOL was able to degrade 16.8 % estradiol. BHAL degraded 30.2 % estradiol. The best results were determined with TTHL (laccase from Thermus thermophilus). Here the percentage of degradation amounted 55.4 %.
The results of the reactions of the laccaes with addition of ABTS are shown in Figure 2. The experimental set ups were the same as the reaction approach without ABTS described above. The X-axis displays the different tested laccases. On the Y-axis the percentages of degraded estradiol (blue) and ethinyl estradiol (red) are shown. The degradation was measured at t0 and after five hours of incubation at 20 °C. The negative control showed no degradation of estradiol. 6.8 % of ethinyl estradiol were decayed. The positive control TVEL0 is able to degrade 100 % estradiol and ethinyl estradiol. The laccase BPUL (from Bacillus pumilus) degraded 46.9 % of used estradiol after ten minutes incubation. ECOL was able to degrade 6.7 % estradiol. BHAL degraded 46.9 % estradiol. With TTHL (laccase from Thermus thermophilus)a degradation 29.5 % were determined.
Spectrofluorophotometer Analysis
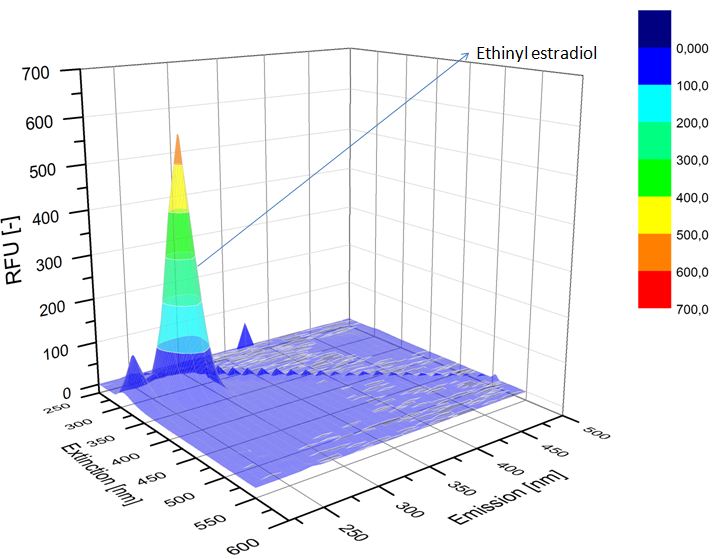
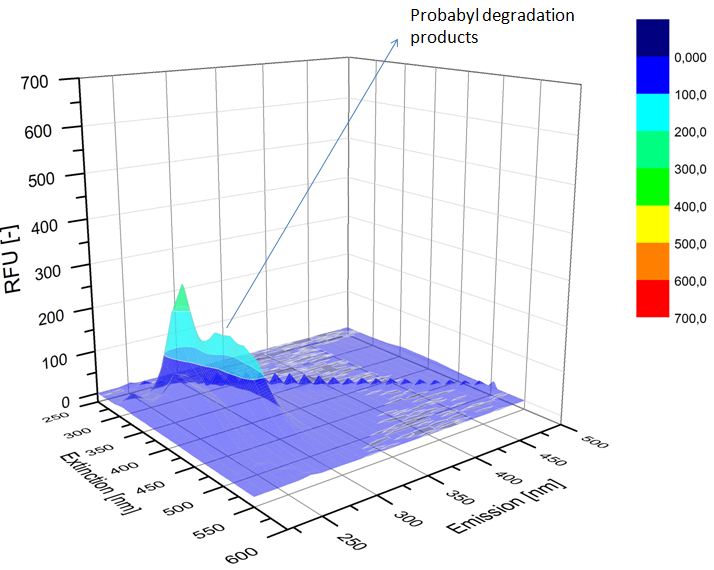
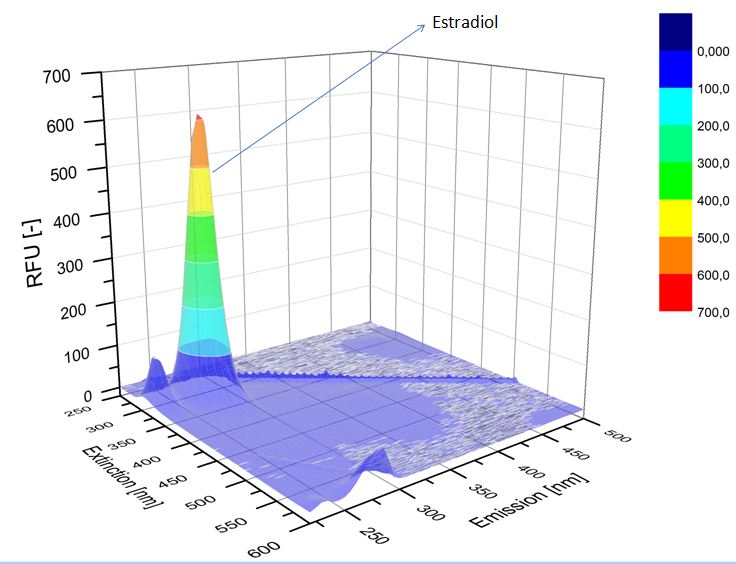
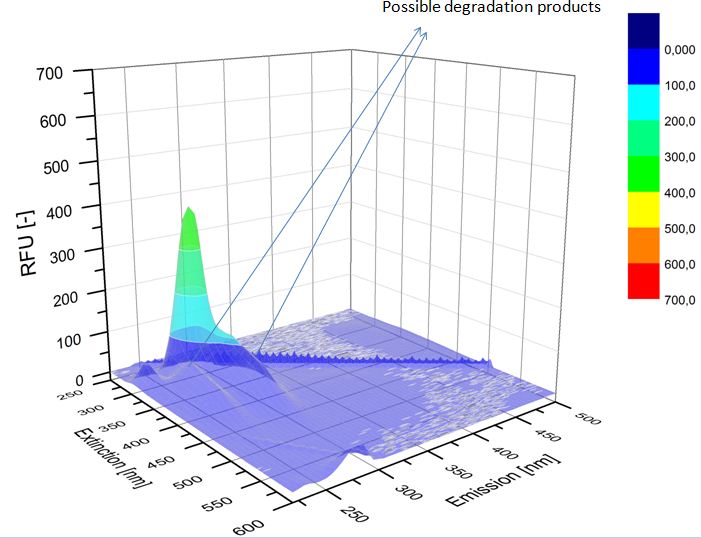
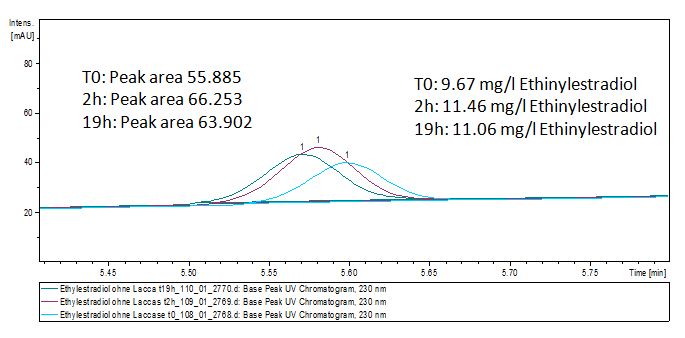
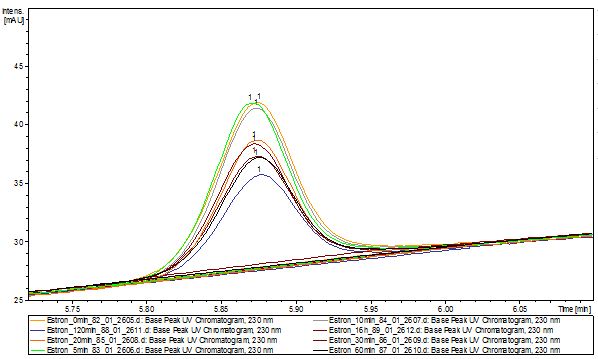
We analyzed the degradation of our substrates with the spectrofluorophotometer. As you can see it in the figures below the ethinyl estradiol and estradiol are degraded over night. Figure 3 shows the ethinyl estradiol without laccase treatment, Figure 4 shows that no more ethinyl estradiol can be detected in the sample after the degradation and new peaks appear, which might be represent possibly degradation products. In Figure 5 you can see the estradiol control without laccases. Like ethinyl estradiol our estradiol peak does not appear after the degradation and new peaks appear indicating that those are new degradation products.
Liquid chromatography–mass spectrometry
Dilution series
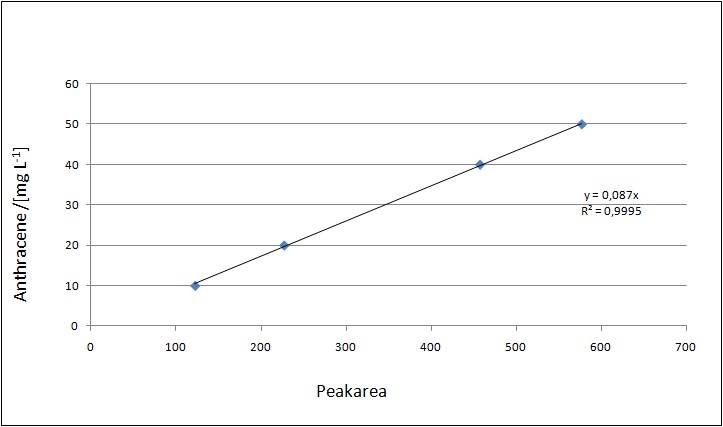
Our substrates are soluble in methanol. We set the standards to a concentration of 1 mg mL-1. The upper detection limit for the LC-MS was evaluated at concentration of 10 µg L-1 for the substrates estrone and estradiol. The same limit of detection was used for ethinyl estradiol and anthracene. We only used those four substrates. For all LC-MS sample preparations we used the T. versicolor laccases. The dilution series was prepared in methanol and 50 % acetonitril-water (v/v).
Degradation results
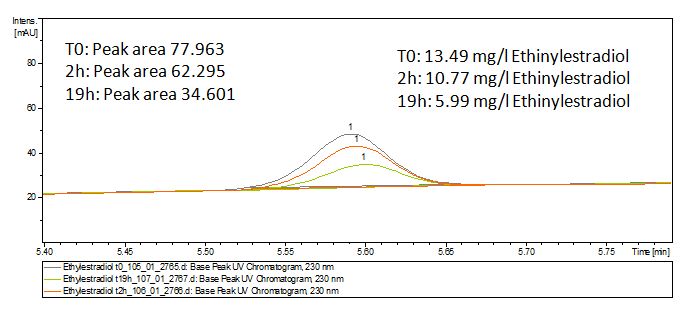
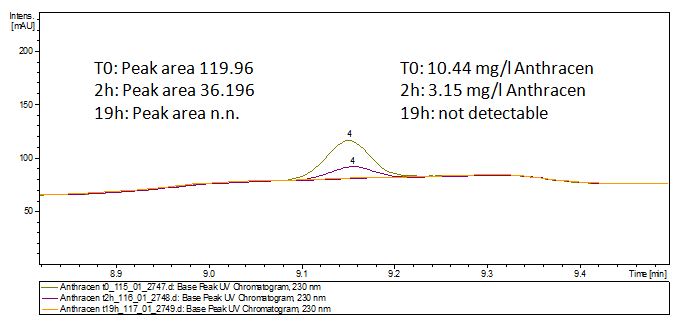
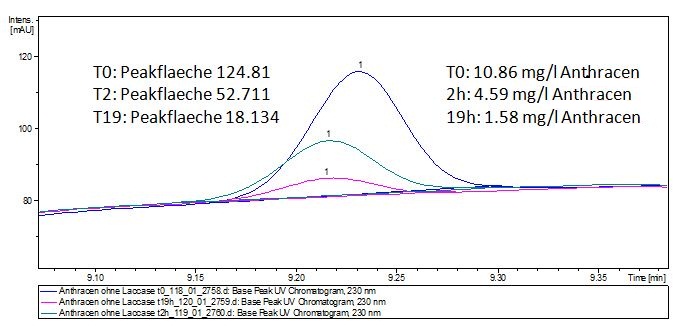
The TVEL0 was able to degrade the synthetic estradiol (Fig. 9) and probably anthracene (Fig. 11). The ethinyl estradiol control showed that it is stable in the used media (Fig. 10). Anthracene disintegrates in the Britton Puffer. But it could be observed, that there is less anthracene measurable with the LC-MS. The results indicate, that the laccase is able to degrade anthracene (Fig. 12). Estrone (Fig. 13) and estradiol (Fig. 14) were degraded as well. Using estrone it could not be identify any degradation products. The reason for this could be that the products are not detectable with LC-MS or with the applied methods. Peaks in the degradation of estradiol have been shown but we were not able to identify them. It could be degradation products. In the following figures the results of the LC-MS measurements are presented.
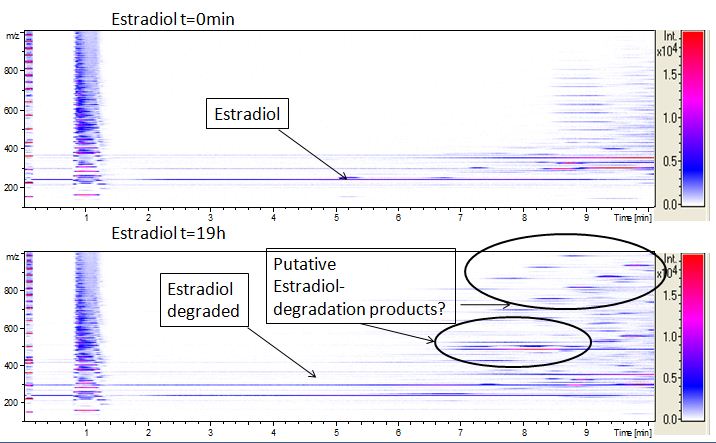
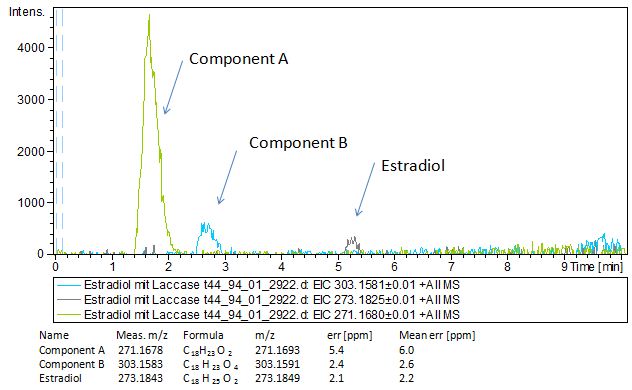
We also tried to measure the degradation using mass-spectrometry. Since quantification via mass-spectrometry is difficult regarding the ionization of the analytes, we quantified our substrates by UV-light. Nevertheless, mass spectrometry enables identification of possible degradation products. We analyzed estradiol degradation in detail (Fig. 14), resulting in the detection of possible chemical compounds generated during the (enzymatic) degradation. Until now we are not sure how estradiol degradation works, but with more time granted, the degradation products can be identified.
Since we have seen some possible degradation products, we used more estradiol, ethinyl estradiol and more laccase for the reaction to the LC-MS measurement. We found out that after the laccase treatment two new peaks appears. A check against databases could not identify thoose components so we did an MS-MS on component A. Since component B is fewer in concentration then component A, we could not find out anything about it (Fig. 15).
| 55px | | | | | | | | | | |
 "
"