Team:Bielefeld-Germany/Results/vector
From 2012.igem.org

Summary
Contents |
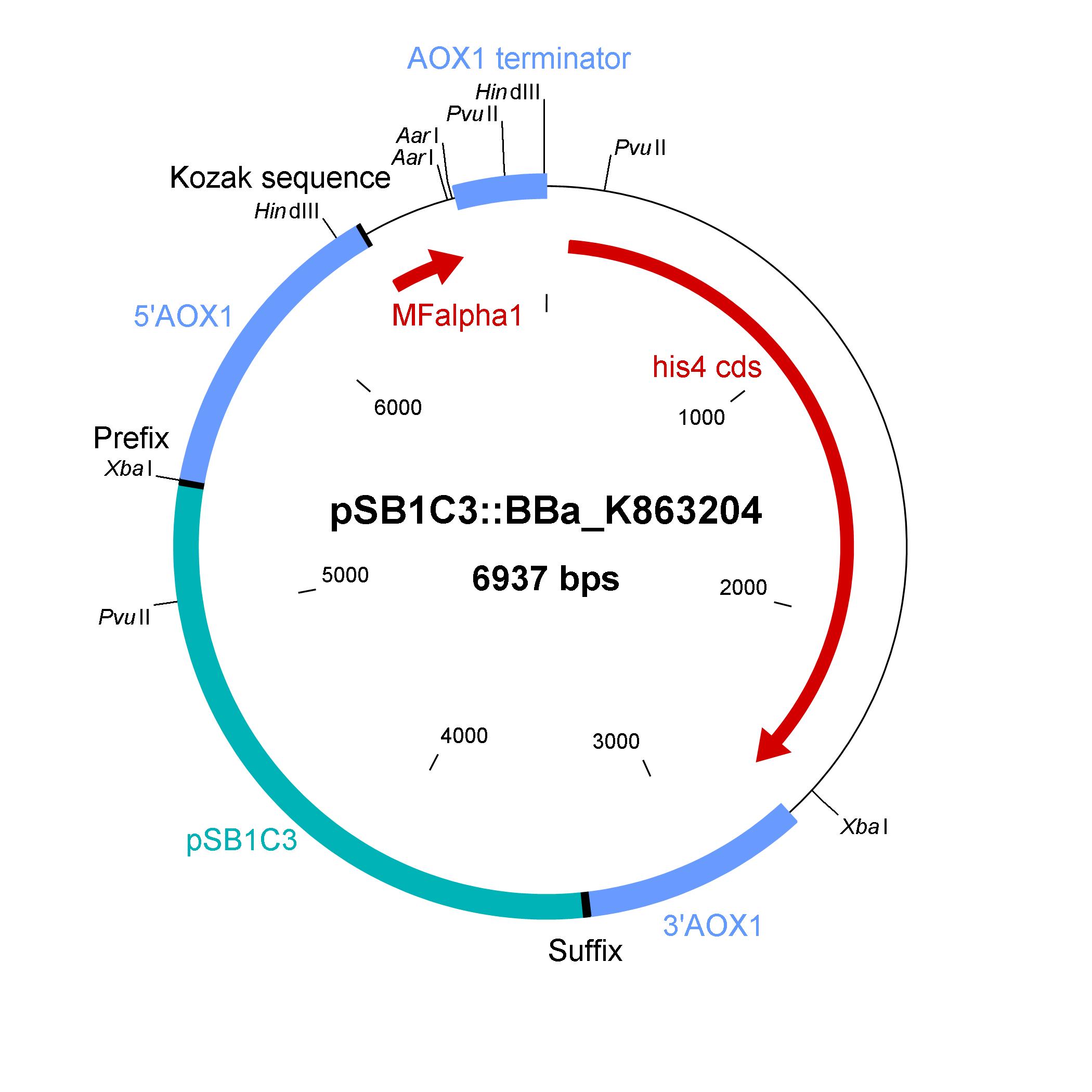
Description of the shuttle vector system
The design of a minimal shuttle vector system with defined regions (or better DNA fragments) for expression and secretion of proteins of interest (POI), like laccase, which needed glycolisation is the basic concept. The shuttle vector needs a bacterial part for cloning in bacteria (like E. coli) and an eucaryotic part for genomic integration and selection in yeast (like P. pastoris). An other team will be able to clime in frame their gene of interest via the AarI restriction site. With only one restriction ligation cloning step the shuttle vector will be ready to use and integrate in the eucaryote P. pastoris.
The shuttle vector consists of the plasmid pSB1C3, 5' UTR of alcohol oxidase 1 gene (aox1) containing the aox1 promoter region, Kozak sequence, mating factor alpha 1 (MFalpha1), AarI restriction site, aox1 terminator, his4 gene and 3' UTR of aox1 gene. Cloning and plasmid replication in E. coli are able via the pSB1C3 part. The gene of interest (like laccase) can be included in frame with MFalpha1 via AarI restriction site. With the N-terminal MFalpha1 the POI could be secreted in the media. Genomic integation of MFalpha1-taged POI is able via the 5' UTR and 3' UTR of the aox1 gene. This allows a double cross over and the genomic integration without any bacterial proportion of DNA which could be a decisive point for industrial application. The complementation of histidine auxotrophie via his4 gene was chosen instead of a zeocine resistance. This selection strategy is chosen because we want to avoid the application of antibiotics.
Gibson assembly was used for building the shuttle vector (see the figure below) and the fragments with 5' overlap were amplified via PCR. In addition, the fragments were designed as basic BioBrick parts for different applications by the community. The origin of the DNA sequence for design of the shuttle vector and the source of DNA for PCR is listed in the table below.
Elements of the shuttle vector and their origin
| Element | BioBrick | Origin of DNA sequence of design | Origin of DNA sequence of PCR |
|---|---|---|---|
| [http://partsregistry.org/Part:pSB1C3 pSB1C3] | [http://partsregistry.org/wiki/index.php/Part:pSB1C3 pSB1C3] | [http://partsregistry.org/Part:pSB1C3 pSB1C3] | [http://partsregistry.org/Part:pSB1C3 pSB1C3] |
| [http://partsregistry.org/Part:BBa_K863200 5'UTR of aox1] | [http://partsregistry.org/Part:BBa_K863200 K863200] | [http://products.invitrogen.com/ivgn/product/V19520 plasmid pPICZalphaA (Invitrogen)] | P. pastoris wild type X-33 |
| Kozak sequence | [http://partsregistry.org/Part:BBa_J63003 J63003] | [http://partsregistry.org/wiki/index.php?title=Part:BBa_J63003 BBa_J63003] | integrated in primer sequence |
| [http://partsregistry.org/Part:BBa_K863203 MFalpha1] | [http://partsregistry.org/Part:BBa_K863206 K863206] | [http://products.invitrogen.com/ivgn/product/V19520 plasmid pPICZalphaA (Invitrogen)] | [http://products.invitrogen.com/ivgn/product/V19520 plasmid pPICZalphaA (Invitrogen)] |
| [http://partsregistry.org/Part:BBa_K863203 aox1 terminater] | [http://partsregistry.org/Part:BBa_K863203 K863203] | plasmid pPIC9K | P. pastoris wild type X-33 |
| [http://partsregistry.org/Part:BBa_K863202 his4] | [http://partsregistry.org/Part:BBa_K863202 K863202] | plasmid pPIC9K | P. pastoris wild type X-33 |
| [http://partsregistry.org/Part:BBa_K863201 3'UTR of aox1] | [http://partsregistry.org/Part:BBa_K863201 K863201] | plasmid pPIC9K | P. pastoris wild type X-33 |
Site directed mutagenesis of the shuttle vector
To remove the illegal XbaI restriction site in the shuttle vector, site directed mutagenesis was done. On the selection medium plattes some clones grow. Colony PCR was done for some of them, but in all tried cases the results are negative.
Shuttle vector in E. coli
The developed shuttle vector could be transformed in E. coli and analyzed by restriction with the enzymes PvuII and HindIII. The restriction pattern is positive. A [http://partsregistry.org/cgi/partsdb/dna.cgi?part_name=BBa%20K863204 sequencing] was also done.
Shuttle vector in P. pastoris
After linearization by restriction with EcoRI and SpeI the shuttle vector could be transformed in competent P. pastoris cells. Transformants grow on selective media plates because of complementation of histidine auxotrophy. For differentiation between the M+ and Ms genotype a PCR with the primer 5AOX-Phenotype-FW and TT-Phenotype-RV have to be done. The M+ phenotype means that a single cross over recombination took place and the cultivation with methanol have no effect on the growth of the cells. The Ms phenotype means that a double cross over recombination took place and the gene aox1 was removed from the genome. During cultivation and induction with methanol the cells grow slow, because now only the gene aox2 exist in the genome.
GFP integrated in shuttle vector
Cloning of green fluorescent protein (GFP) via the AarI restriction site into the shuttle vector was done to test the system. A restriction analysis show that the construction were successful. The site directed recombination failed and no clones could be found.
TVEL5 integrated in shuttle vector
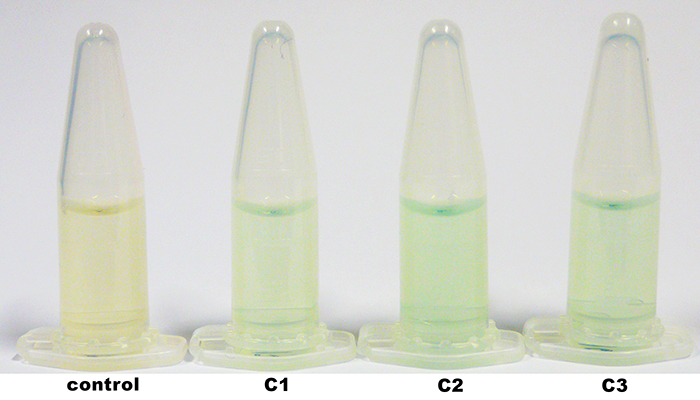
The laccase TVEL5 was cloned via the AarI restriction site into the shuttle vector ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K863207 BBa_K863207]). Restriction analysis and sequencing confirm that success. Furthermore the linearized construct could transformed in the yeast P. pastoris, that could be shown by PCR. The construct was integrated into the genome with a single cross over. This results in a M+ phenotype. In addition, although the production and secretion of the active laccase TVEL5 after induction with methanol worked.
| 55px | | | | | | | | | | |
 "
"