Team:HokkaidoU Japan/Notebook/aggregation Week 5
From 2012.igem.org
Contents |
July 30th
digestion
I tried to digest the mini-prep products again, and after Ethanol precipitation, we did electrophoresis.
transformation
I think that the E. coli which we use transformation is BL21(DE3)pLysS.
So, the E. coli could multiplication increase on LBC plate.
I'm going to do transformation , use DH5α.
Transformation of plasmid DNA ligated in 25th (pT7-RBS-Ag43-dT on pSB1K3) in E. coli(DH5α).
- Added 2ul of plasmid DNA to 50ul of thawed competent cells on ice.
- Incubated on ice for 30min.
- Added 200ul of LB then incubated the cells for 2 hours at 37C.
- Prepared and Labeled two petri dishes with LBK.
- Plate 200ul of the transformation onto first dish and spread.
- Added 450ul of LB to 50ul of the transformation and plated 200ul of it onto second dish and spread.
- Incubated the plates at 37C for 14 hours.
July 31th
liquid culture
Liquid culture for pT7-RBS-Ag43-dT on pSB1K3.
- Added 2ml of LBK into culture tubes.
- Resuspended colonies.
- Incubated the tubes at 37C for
August 1st
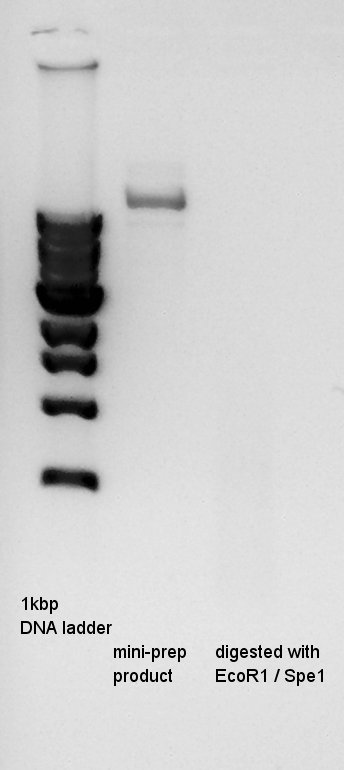
mini-prep
mini-prep of pT7-RBS-Ag43-dT which had been cultivated from yesterday. We used FastGene Plasmid Mini Kit(Nippon Genetics) and got 50ul of DNA solutions. [[image:|thumb|mini-prep result]]
Digestion
pT7-RBS(20ng/ul) =⑨ SpeI 10xH
| DNA solution | 4.5ul |
| SpeI | 1ul |
| 10xH buffer | 1ul |
| DW | 3.5ul |
| Total | 10ul |
SpeI 10xM
| DNA solution | 4.5ul |
| SpeI | 1ul |
| 10xM buffer | 1ul |
| DW | 3.5ul |
| Total | 10ul |
pT7-RBS(30ng/ul) =⑩
SpeI 10xH
| DNA solution | 3ul |
| SpeI | 1ul |
| 10xH buffer | 1ul |
| DW | 5ul |
| Total | 10ul |
SpeI 10xM
| DNA solution | 3ul |
| SpeI | 1ul |
| 10xM buffer | 1ul |
| DW | 5ul |
| Total | 10ul |
[[image:|thumb|digestion result]]
We couldn't cut them exactry so we cut once more time.
pT7-RBS(20ng/ul) =⑨ SpeI 10xH
| DNA solution | 4.5ul |
| SpeI | 1ul |
| 10xM buffer | 2ul |
| DW | 12.5ul |
| Total | 20ul |
Liquid culture
Liquid culture for pBAD-RBS on pSB1K3.
- Added 2ml of LBK into culture tube.
- Scraped the surface of glycerol stock of construct.
- Incubated the tube at 33C for OOhrs.
July 30th
Preparing chemical competent cell
Preparing chemical competent cell of BL21.
Our competent cell Protocol
- Single colony isolation on LB plate
- incubate the plate for 15-19 hours at 37℃
- lift colony of E.coli into 2 ml LB
- culture cells at 37℃ for 12-16 hours at 180-200 rpm
- transfer 30 ul, 100 ul, 300 ul of the culture into 100 ml of SOB medium, respectively
- culture cells at 20℃ (for 24 hours over) at 180-200 rpm (to ΔOD550nm=0.5~0.6)
- leave the 300 ml flask for 10 min on ice
- transfer the culture into two 50 ml Falcon tube
- centrifuge 3000 rpm at 4℃ for 20 min (TOMY LX-120 rotor), and discard sup
- suspend the pellet in ice-cold 15 ml of TB (Transformation Buffer)(7.5 ml/tube)
- collect them in one tube
- centrifuge 3000 rpm at 4℃ for 20 min (TOMY LX-120 rotor), and discard sup
- suspend the pellet in ice-cold 3.2 ml of TB (Transformation Buffer)
- Instil 0.24 ml of DMSO in precipitant
- leave the 50 ml Falcon tube for 10 min on ice
- divide 50ul of solutions in each 1.5 or 0.5 ml tubes
- Freeze the suspension in liquid nitrogen
- store at –80℃
 "
"