Team:HokkaidoU Japan/Notebook/aggregation Week 5
From 2012.igem.org
(Difference between revisions)
| Line 8: | Line 8: | ||
==digestion== | ==digestion== | ||
<p> | <p> | ||
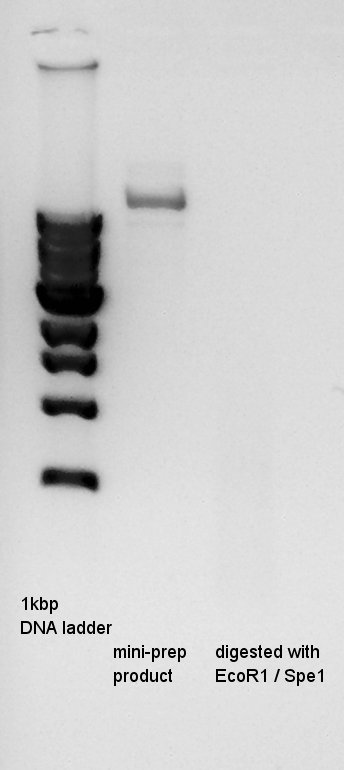
| - | I tried to | + | I tried to digest the mini-prep products again, and after Ethanol precipitation, we did electrophoresis. |
[[image:HokkaidoU2012_120730_ag43-f1-e-s.jpg|thumb|digestion result]] | [[image:HokkaidoU2012_120730_ag43-f1-e-s.jpg|thumb|digestion result]] | ||
| Line 25: | Line 25: | ||
#Plate 200ul of the transformation onto first dish and spread. | #Plate 200ul of the transformation onto first dish and spread. | ||
#Added 450ul of LB to 50ul of the transformation and plated 200ul of it onto second dish and spread. | #Added 450ul of LB to 50ul of the transformation and plated 200ul of it onto second dish and spread. | ||
| - | #Incubated the plates at 37C for | + | #Incubated the plates at 37C for 14 hours. |
</p> | </p> | ||
Revision as of 07:54, 31 July 2012
Contents |
July 30th
digestion
I tried to digest the mini-prep products again, and after Ethanol precipitation, we did electrophoresis.
transformation
I think that the E. coli which we use transformation is BL21(DE3)pLysS.
So, the E. coli could multiplicationincrease on LBC plate.
I'm going to do transformation , use DH5α.
Transformation of plasmid DNA ligated in 25th (pT7-RBS-Ag43-dT on pSB1K3) in E. coli(DH5α).
- Added 2ul of plasmid DNA to 50ul of thawed competent cells on ice.
- Incubated on ice for 30min.
- Added 200ul of LB then incubated the cells for 2 hours at 37C.
- Prepared and Labeled two petri dishes with LBK.
- Plate 200ul of the transformation onto first dish and spread.
- Added 450ul of LB to 50ul of the transformation and plated 200ul of it onto second dish and spread.
- Incubated the plates at 37C for 14 hours.
July 30th
liquid culture
Liquid culture for pT7-RBS-Ag43-dT on pSB1K3.
- Added 2ml of LBK into culture tubes.
- Resuspended colonies.
- Incubated the tubes at 37C for
 "
"