Team:HokkaidoU Japan/Notebook/Week 1
From 2012.igem.org
(→July 2nd) |
(→July 2nd) |
||
| Line 5: | Line 5: | ||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
==July 2nd== | ==July 2nd== | ||
| - | < | + | <div> |
On your mark... | On your mark... | ||
| - | </ | + | </div></div> |
Revision as of 06:25, 16 July 2012
Contents |
July 2nd
On your mark...
July 3rd
Get set...
July 4th
Transformation
Transformation of BBa_B0015(dT), B0034(RBS), I179005(pT7), K346007(Ag43), and K542009(pLacI-RBS-Ag43) in DH5α
- Added each DNA solutions (1ul) into DH5α comeptent cell and standed on ice in 30 min.
- Cultivated on LBA(dt,RBS,T7) and LBC(Ag43, pLacI-RBS-Ag43). E.coli cultivate in LBC was pre-cultivated in 2 hrs. pT7 was 21hrs and Others were 20hrs cultivated
July 5th
Transformation
K346007(Ag43) was failed to cultivate on LBC plate. Transformation of K346007(Ag43) in DH5α.
- Add DNA solution(1ul) into DH5α comeptent cell and stand on ice in 30 min.
- Pre-cultivated in 2hrs.
- Cultivated on LBC in 21hrs.
Single colony isolation
Single colony isolation of BBa_B0015, B0034, I179005 and K542009.
- Picked up one colony.
- Cultivation on LBA(dt,RBS,T7) and LBC(pLacI-RBS-Ag43) in 14hrs30mins
July 6th
- Liquid culture
- Picked up two colonies from each plates.
- One colony was dipped in 1ml LB (A or C), and other colony was dipped in 2ml LB(A or C). 1ml is for glycerol stocks and 2ml is for mini-prep.
- 16hrs Cultivation
- Single colony isolation
- Single colony isolation of K346007(Ag43).
July 7th
Liquid culture
Liquid culture in LBC(Ag43).
- Picked up two colonies from each plates.
- Both of colonies were dipped in 2ml LBC, and then we cultivate them in 38℃.
However, one of them cultivated only 8 hours. It's for glycerol stock.
3A assembly
Assembled pT7, RBS and pSB1C3 by 3A assembly. This 3A assembly is our first try!
mini-prep
- mini-prep of dT,RBS,pT7 and pLacI-RBS-Ag43.
- Elution in 50ul buffer
Glycerol stock
Made glycerol stocks of dT,RBS,pT7 and pLacI-RBS-Ag43.
- Parts written above were cultivated in LBA(dT,RBS,pT7) and LBC(pLacI-RBS-Ag43) 1ml in 16hrs 30min.
- Add glycerol and Freeze at -80C
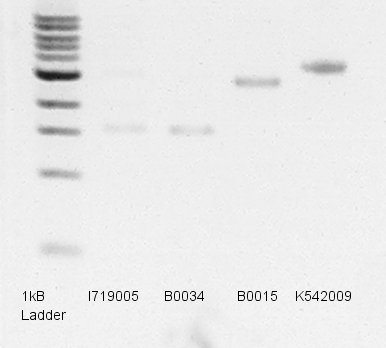
Electrophoresis
Electrophoresis to predict concentration of mini-prep products(dT,RBS,pT7 and pLacI-RBS-Ag43).
- Used 1% agarose gel.
- Pre-migration.
- Migrated 1.2ul of DNA solutions (1ul is mini-prep products and 0.2ul is Loading Dye) in 35min.
- Took a photograph of 1% agarose gel that finished electrophoresis.
Digestion
Digestion of I719005, B0034 and pSB1K3
Digestion recipe
All parts were reacted in 30ul solution.
- I719005(40ng/ul)
| DNA solution | 12.5ul |
| EcoRI | 1ul |
| SpeI | 1ul |
| 10xH Buffer | 3ul |
| DW | 12.5ul |
- B0034(40ng/ul)
| DNA solution | 12.5ul |
| XbaI | 1ul |
| PstI | 1ul |
| 10xM Buffer | 3ul |
| DW | 12.5ul |
- pSB1K3(25ng/ul)
| DNA solution | 12ul |
| EcoRI | 1ul |
| PstI | 1ul |
| 10xH Buffer | 3ul |
| DW | 13ul |
Ethanol precipitation
For rising concentration of DNA solution which use for Ligation and removing restriction enzyme.
- Added 3ul of NaoAc, 1.5ul of glycogen and 75ul of 100% ethanol.
- Centrifuged in 14000rpm, 30min at 4C.
- Remove supernatant and added 220ul of 70% ethanol.
- Centrifuged in 15000rpm, 15min at 4C.
- Remove supernatant and air drying in room temperature then added 10ul of DW.
Ligation
All DNA solutions were digested. 3A assembly protocol required Ligation reaction should be in total 25ul solution.
| Ligation Mighty Mix | 12.5ul |
| pT7 | 2ul |
| RBS | 2ul |
| pSB1K3 | 2ul |
| DW | 6.5ul |
| Total | 25ul |
Ligation reaction recipe was written below.
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
ligation was finished.
But now pm10:00. 2.5hrs needs for doing transformation. Transformation would finish at am:0:30.
Withdraw!!!!
July 8th
- (pT7 + RBS)
- Transformation
- Added DNA soltions (Ligation products) 1ul to DH5α compitent cell.
- Stood on ice in 30min.
- Added 600ul of LB to transformed DH5α solution.
- Pre-cultivate in 2hrs
- Splead 300ul of LB&DH5α solution to LBK.
- Cultivated
- K346007(Ag43)
- mini-prep
- Used FastGene Plasmid Mini Kit(Nippon Genetics)
- Elutioned in 50ul
- First we eluted in colection tube. then moved in Eppendorf tube.
- Erectrophoresis
- Prepared 1% Agalose gel and added EtBr then pre-migration in 30min.
- 1ul 1kb ladder, 1.2ul mini-prep product(1ul is DNA solution and 0.2ul is loading dye) added then migtrated
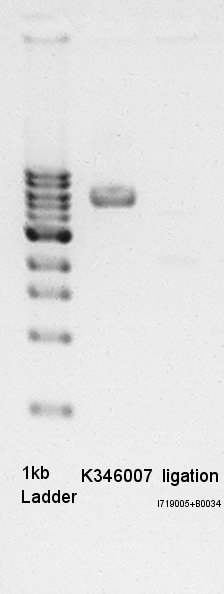
- Glycerol stock
- Parts written above were cultivated in LBC.
- Added glycerol and Freezed at -80C
- (Ag43 + dT)
- Digestion
- Ag43(Insert)
| DNA solution | 48ul |
| EcoRI | 1ul |
| SpeI | 1ul |
| 10xH buffer | 6ul |
| 4ul | |
| Total | 60ul |
- dT(Vector)
3318bp(Ag43 + pSB1AK3)
| DNA solution | 8ul |
| EcoRI | 1ul |
| XbaI | 1ul |
| 10xM buffer | 2ul |
| DW | 8ul |
| Total | 20ul |
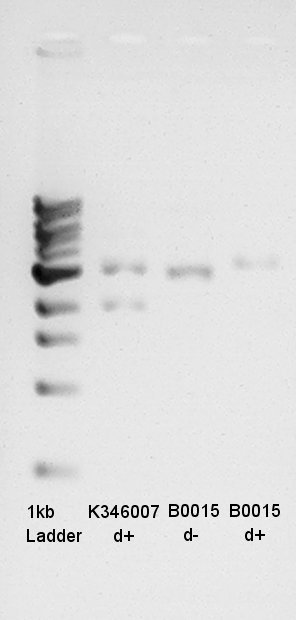
Digestion result image
K346007(Ag43) was 5190bp before digestion (Biobrick prefix + Ag43 + Biobrick suffix + pSB1C3).
After digestion, Ag43 and pSB1C3 was separated and became fragments about 3120bp(Ag43) and 2070bp(pSB1C3).
Gel image above shows there are two fragments, one fragment is about 2000bp other fragment is 3000bp.
Digestion would be succeeded.
About Vector(Boo15:dT), the DNA was circular so DNA migrated more far than Linear DNA. d+ (Circular DNA) migrated little more far than d- (Linear DNA). so we think digestion was succeeded.
- Ethanol precipitation
Ethanol precipitation for gel extraction products(K346007(Ag43) and B0015(dT))
- Added 5ul of NaoAc , 1.5ul of glycogen and 125ul of 100% ethanol to 50ul DNA solutions.
- Centrifuged in 15000rpm, 10min at 4C.
- Remove supernatant and added 220ul of 70% ethanol.
- Centrifuged in 15000rpm, 5min at 4C.
- Remove supernatant and air drying in room temperature then added 10ul of DW.
- Ligation
All DNA solutions were digested. Used Ligation Mighty Mix(TakaraBio)
| Ligation Mighty Mix | 5ul |
| Insert: Ag43 | 2ul |
| Vector: dT | 2ul |
| DW | 1ul |
| Total | 10ul |
Ligation reaction recipe was written below.
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
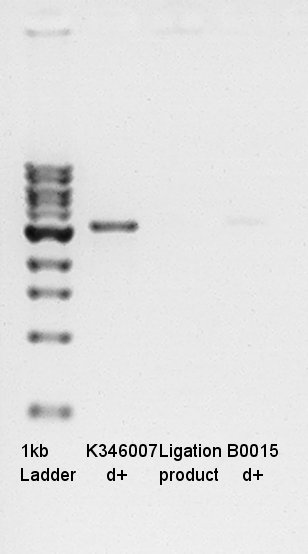
- Electrophoresis
Confirmation of succession of ligation.
- Prepared 1% Agalose gel and added EtBr then pre-migration in 30min.
- Added 1kb ladder, Ligation product(1ul) and digestion products (control:each solutions 1ul).
- Migtrated in 30min.
Electrophoresis results
- Transformation
Transformation for K346007(Ag43)+B0015(dT) on pSB1AK3.
- Added DNA soltions (Ligation products) 1ul to DH5α compitent cell.
- Stood on ice in 30min.
- Added 600ul of LB to transformed DH5α solution.
- From 700 solution(100ul is DH5α and 600ul is LB), 100ul add to 900ul of LB(x10 solution)
- Spread 300ul from 600(700-100)ul and 1000ul of LB&DH5α solution to each LBA plates.
- Cultivated.
 "
"