Team:Bielefeld-Germany/Results/comparison
From 2012.igem.org
(→Immobilization) |
(→Immobilization) |
||
| Line 49: | Line 49: | ||
After the successful immobilization, the second step was to test the activity of the immobilized laccases. The specific enzyme activity of immobilized laccases from ECOL and BPUL was measured using [https://2012.igem.org/Team:Bielefeld-Germany/Protocols/Analytics ABTS as substrate] and compared to that of nonimmobilized laccases (Fig. 7). The results showed that immobilized BPUL preserved 44 % of its activity, whereas ECOL didn't show a significant activity after immobilization. However, further tests using "Thermo Biomate 3 UV-Vis Spektrophotometer" showed a 21% activity of ECOL after immobilization (results not shown). | After the successful immobilization, the second step was to test the activity of the immobilized laccases. The specific enzyme activity of immobilized laccases from ECOL and BPUL was measured using [https://2012.igem.org/Team:Bielefeld-Germany/Protocols/Analytics ABTS as substrate] and compared to that of nonimmobilized laccases (Fig. 7). The results showed that immobilized BPUL preserved 44 % of its activity, whereas ECOL didn't show a significant activity after immobilization. However, further tests using "Thermo Biomate 3 UV-Vis Spektrophotometer" showed a 21% activity of ECOL after immobilization (results not shown). | ||
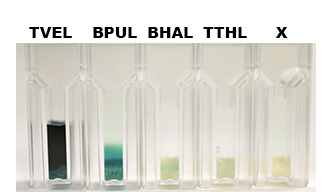
| - | [[File:Bielefeld2012-Beadsbild.jpg|500px| | + | [[File:Bielefeld2012-Beadsbild.jpg|500px|right|thumb|'''Fig. 8: Enzymatic activity of the purified laccases BPUL, BHAL and TTHL illustrated by the oxidation of ABTS.''' BPUL shows a very high activity whereas the activity of BHAL and TTHL isn't so high, probably due to the low laccase concentration (4 μg ml<sup>-1</sup>). Yet, BHAL shows a higher activity than TTHL.]] |
Revision as of 03:30, 27 October 2012

Activity Tests
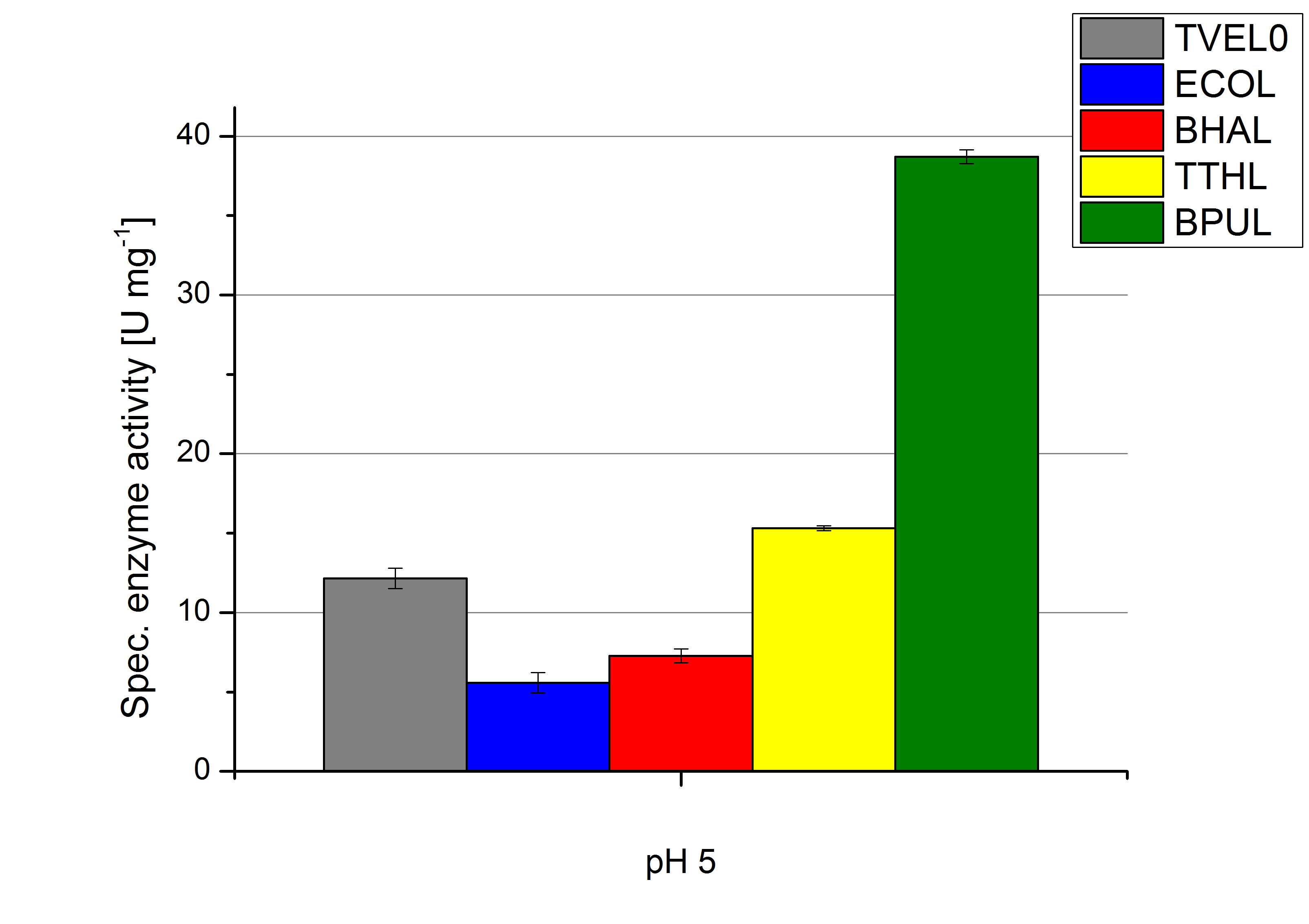
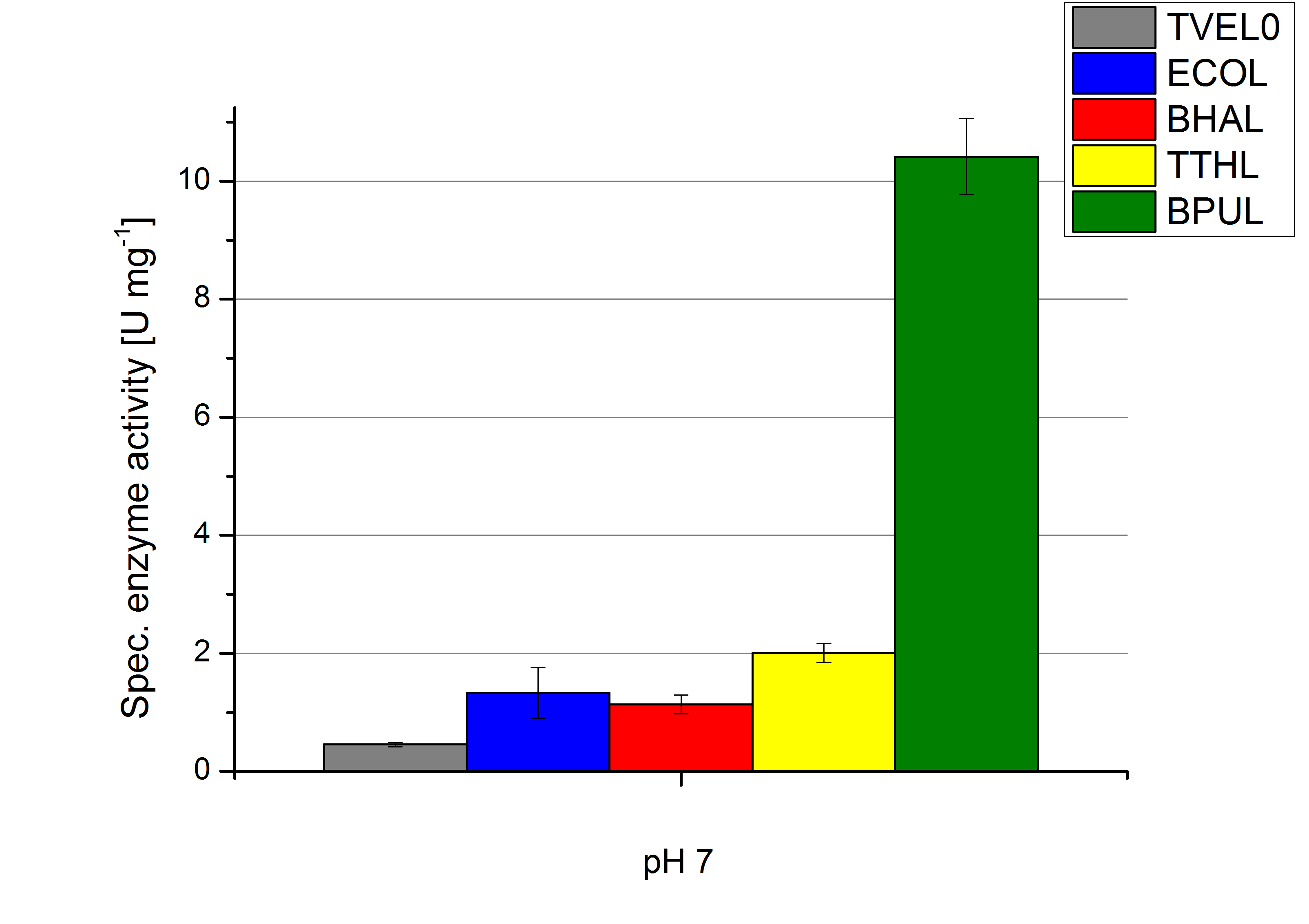
To optimize the application of our enzymes [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863000 BPUL], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863005 ECOL], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863022 BHAL] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863010 TTHL] in a waste water treatment plant the obtained results were compared and further analyzed. All laccases show an optima at pH 5. However BPUL distinguishes from the other enzymes by showing a significantly higher enzyme activity. At pH 7 respectively the maximal activity is as well seen in the BPUL sample. At pH 5 ECOL, BPUL, BHAL, TTHL and TVEL0 show an average activity of 10-15 U mg-1. Though at pH 7 the enzymes activities were calculated to not exceed 2 U mg-1, respectively. This indicates that at pH values of around 7 BPUL is the best choice for our filter and if other enzymes are desired or needed an higher amount has to be used.
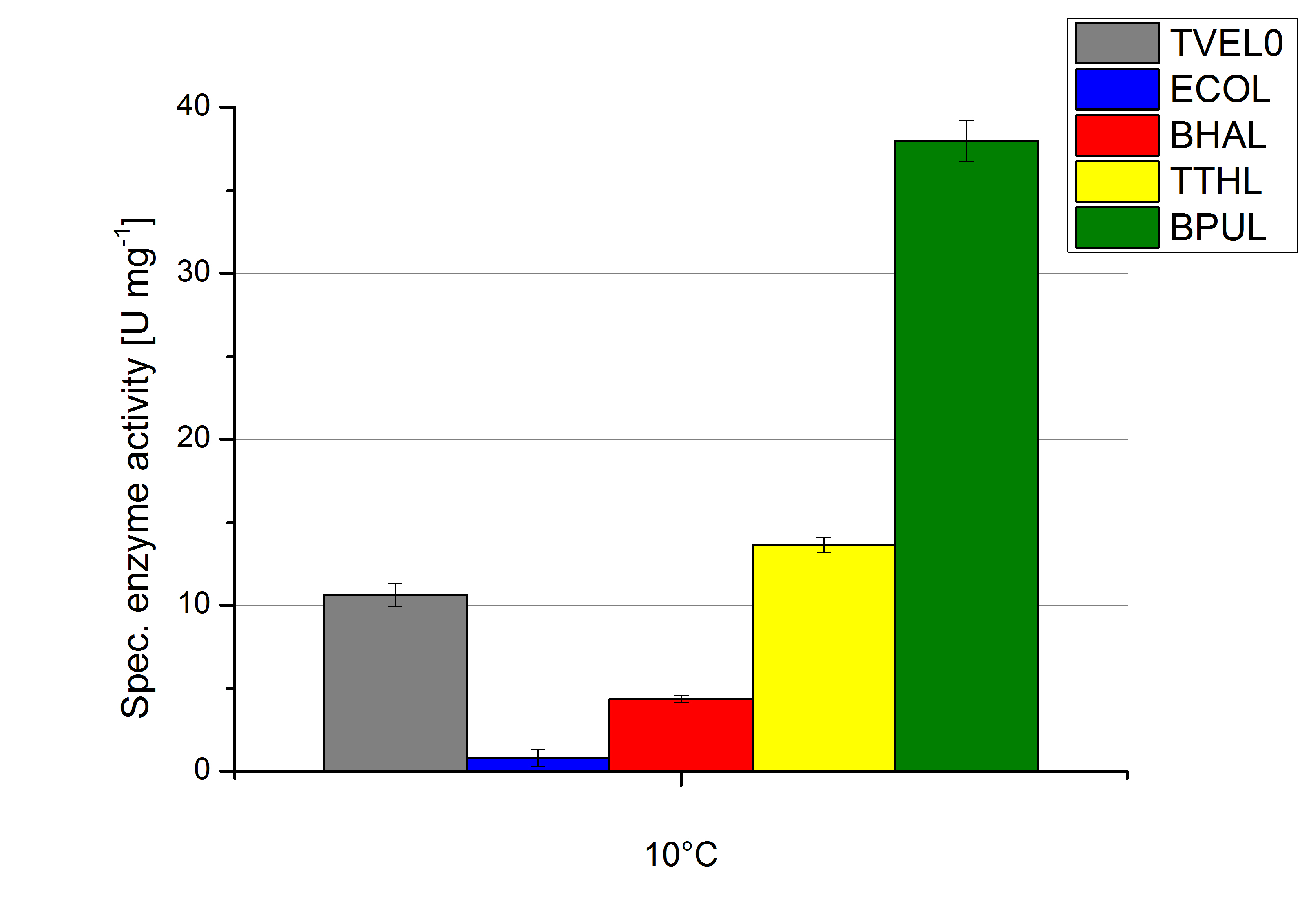
Regarding the temperature characterizations BPUL is observed to show an enzyme activity of 37 U mg-1. ECOL, BPUL, BHAL, TTHL and TVEL0 show an activity of 2 to 13 U mg-1. Thus BPUL and also TTHL are very feasible for an application even in cold environments. For a usage of BHAL and ECOL a longer incubation time of the laccases and the substrates needs to take place to ensure a proper degradations of microcontaminents.
Substrate Analysis
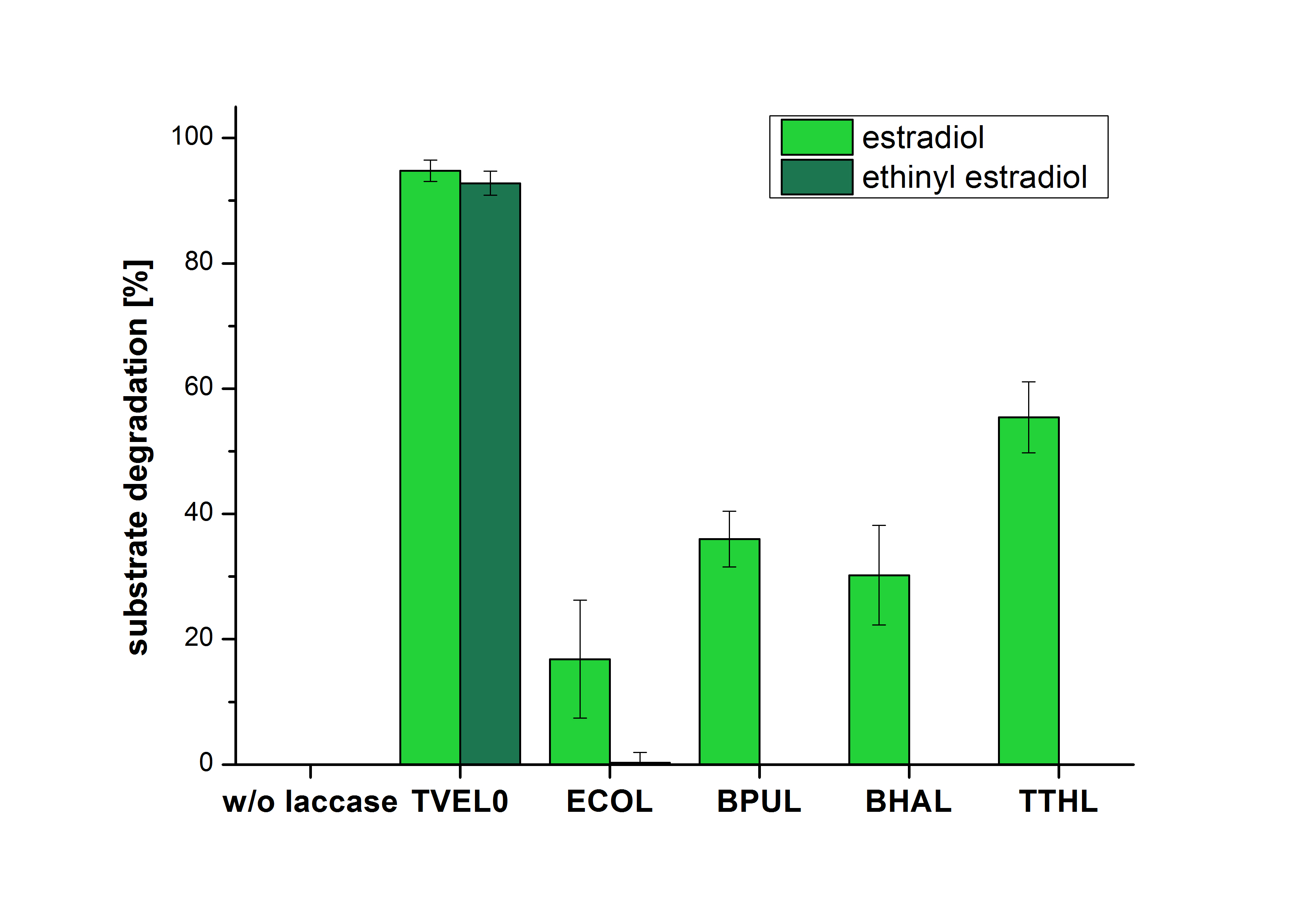
The measurements were made to test whether the produced laccases were able to degrade different hormones. Therefore the produced laccases were inserted in the same concentrations (3 µg mL-1) to the different measurement approaches. To work with the correct pH value (which were measured by the Team Activity Test) Britton Robinson buffer at pH 5 was used for all measurements. The initial substrate concentration was 5 µg mL-1. The results of the reactions without ABTS are shown in Figure 4. On the Y-axis the percentages of degraded estradiol (blue) and ethinyl estradiol (red) are indicated. The X-axis displays the different tested laccases. The degradation was measured at t0 and after five hours of incubation at 30 °C. The negative control was the substrate in Britton Robinson buffer and showed no degradation of the substrates. The bought laccase TVEL0 which is used as positive control is able to degrade 94.7 % estradiol and 92.7 % ethinyl estradiol. The laccase BPUL (from Bacillus pumilus) degraded 35.9 % of used estradiol after five hours. ECOL was able to degrade 16.8 % estradiol. BHAL degraded 30.2 % estradiol. The best results were determined with TTHL (laccase from Thermus thermophilus). Here the percentage of degradation amounted 55.4 %.
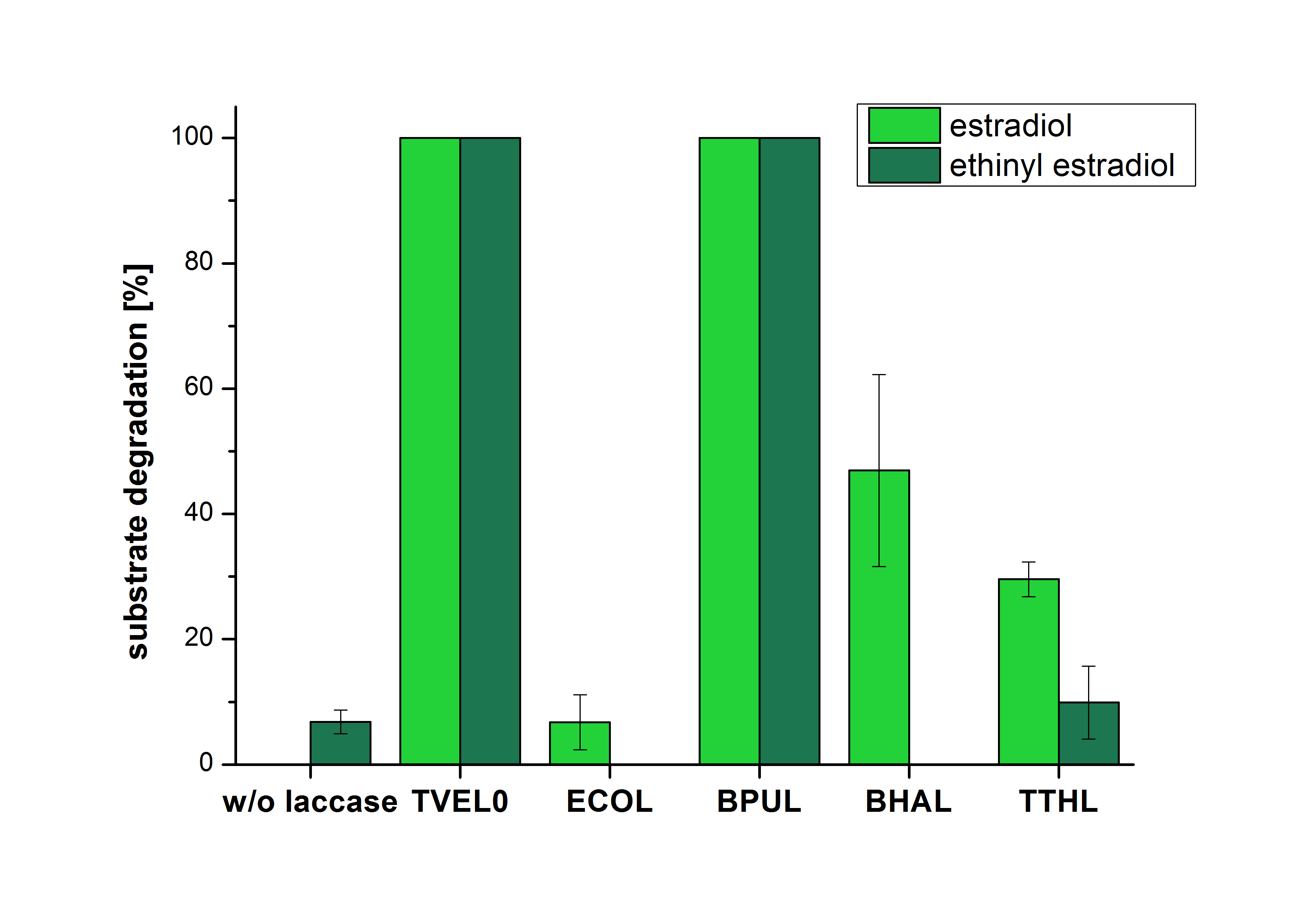
The results of the reactions of the laccases with addition of ABTS are shown in Figure 5. The experimental set ups were the same as the reaction approach without ABTS described above. The X-axis displays the different tested laccases. On the Y-axis the percentages of degraded estradiol (blue) and ethinyl estradiol (red) are shown. The degradation was measured at t0 and after five hours of incubation at 20 °C. The negative control showed no degradation of estradiol. 6.8 % of ethinyl estradiol was decayed. The positive control TVEL0 is able to degrade 100 % estradiol and ethinyl estradiol. The laccase BPUL (from Bacillus pumilus) degraded 46.9 % of used estradiol after ten minutes incubation. ECOL was able to degrade 6.7 % estradiol. BHAL degraded 46.9 % estradiol. With TTHL (laccase from Thermus thermophilus) a degradation 29.5 % were determined.
Immobilization
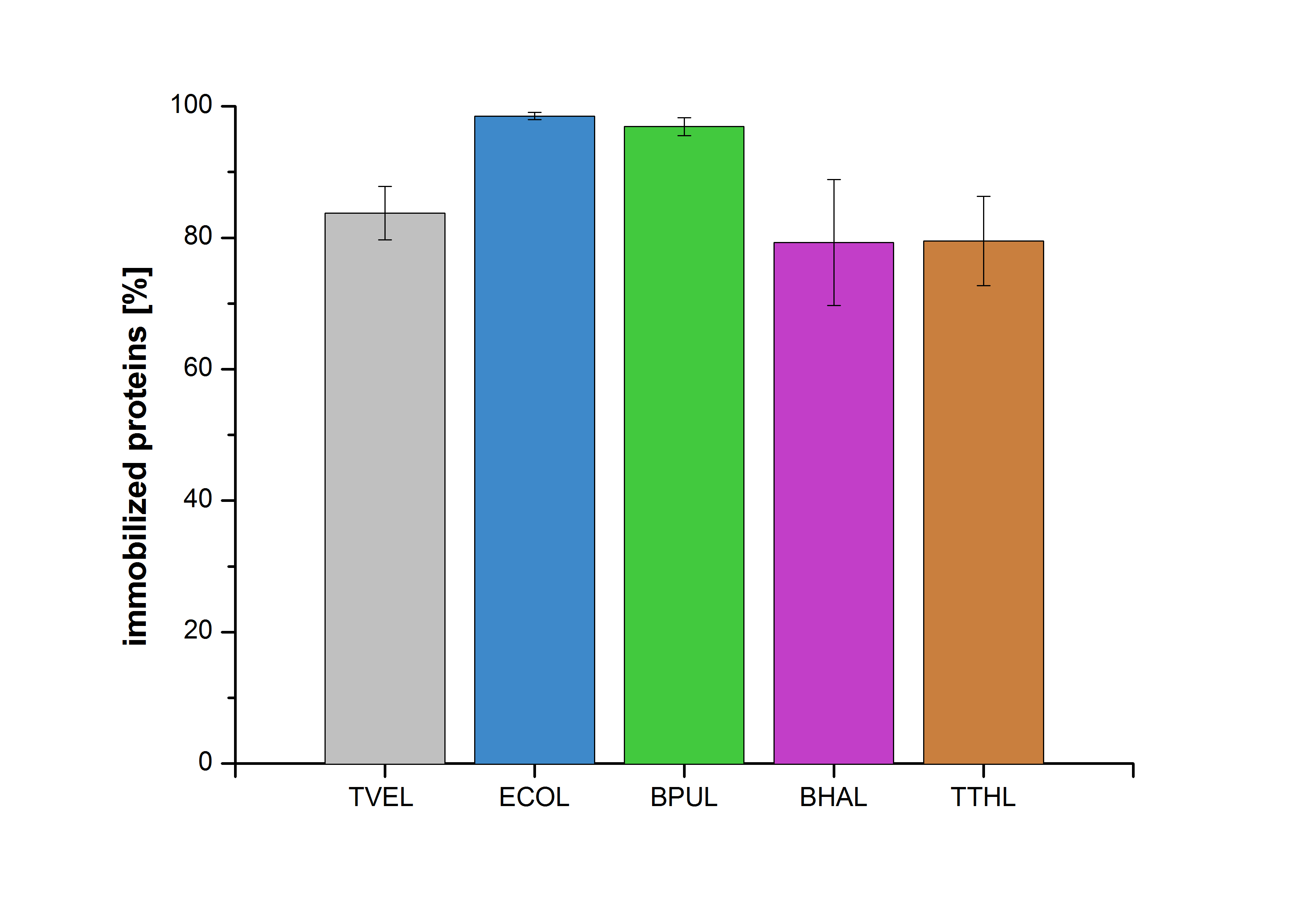
An immobilization experiment was carried out using TVEL0, purified laccases from Escherichia coli BL21 DE3 (named ECOL), [http://www.dsmz.de/catalogues/details/culture/DSM-27.html|thumb|300px|left| Bacillus pumilus DSM 27 (ATCC7061)] (named BPUL), [http://www.dsmz.de/catalogues/details/culture/DSM-18197.html?tx_dsmzresources_pi5 Bacillus halodurans C-125 ] (named BHAL) and from [http://www.dsmz.de/catalogues/details/culture/DSM-7039.html?tx_dsmzreso Thermus thermophilus HB27] (named TTHL) with a bead concentration of 0.12 g and over an incubation period of 14 hours. The concentration of laccases in the supernatant after incubation was measured using Roti®-Nanoquant: The results showed that the four different purified laccases bind very well to the beads. ECOL and BPUL were perfectly immobilized (99% and 97% respectively) and showed a higher binding ability than the standard laccase TVEL0. 79 % of BHAL and TTHL could be immobilized on the CPC-beads (see Fig. 6).
After the successful immobilization, the second step was to test the activity of the immobilized laccases. The specific enzyme activity of immobilized laccases from ECOL and BPUL was measured using ABTS as substrate and compared to that of nonimmobilized laccases (Fig. 7). The results showed that immobilized BPUL preserved 44 % of its activity, whereas ECOL didn't show a significant activity after immobilization. However, further tests using "Thermo Biomate 3 UV-Vis Spektrophotometer" showed a 21% activity of ECOL after immobilization (results not shown).
Nevertheless, due to the variable concentration of the four purified laccases, it wasn't possible to achieve a reasonable comparison in their activity after immobilization. The concentration of purified BHAL and TTHL was too low (4 μg ml-1) to yield comparable results. Yet, all laccases showed indeed an activity (see Fig. 8)
| 55px | | | | | | | | | | |
 "
"