Team:NTU-Taida/Project/Background
From 2012.igem.org
(→Complex Adaptive BioSystems) |
(→Complex Adaptive BioSystems) |
||
| Line 4: | Line 4: | ||
Human bodies are highly fluctuating complex systems. They detect and integrate the clues from changing environments and their own internal states, making numerous responses after delicate computation and regulation. Traditional routes of drug administration including '''oral intake''' and '''intravenous injection''' may be too simplified to promptly fit the real-time condition of the body states. In addition, the frequent and repetitive intake of drugs may be annoying, and sometimes the invasive processes are afflictive, bringing inconvenience to our daily lives. Medical instruments or electrical monitors can instantaneously detect and response to some specific physiological or pathological parameters, but they are usually too heavy and bulky to carry, which restrict the mobility of patients while using it. Therefore we aim to program the intestinal microbes to build our novel '''smart drug delivery systems—PEPDEX'''. | Human bodies are highly fluctuating complex systems. They detect and integrate the clues from changing environments and their own internal states, making numerous responses after delicate computation and regulation. Traditional routes of drug administration including '''oral intake''' and '''intravenous injection''' may be too simplified to promptly fit the real-time condition of the body states. In addition, the frequent and repetitive intake of drugs may be annoying, and sometimes the invasive processes are afflictive, bringing inconvenience to our daily lives. Medical instruments or electrical monitors can instantaneously detect and response to some specific physiological or pathological parameters, but they are usually too heavy and bulky to carry, which restrict the mobility of patients while using it. Therefore we aim to program the intestinal microbes to build our novel '''smart drug delivery systems—PEPDEX'''. | ||
| - | There are around \(10^{13}\) to \(10^{14}\) microorganisms inhabiting in our gastrointestinal tracts, more than 10 times that of the total number of cells in human bodies. They consist of more than 1000 species, and contain 150 times as many genes as our genomes. Colonizing soon after our birth, these microbes comprise a huge community and closely interact with their hosts, having great influence on our '''immune systems''', '''endocrine''', '''metabolic states''', and even '''nervous systems''', from birth to death, from health to illness. They seem to be tiny natural machines that can be | + | There are around \(10^{13}\) to \(10^{14}\) microorganisms inhabiting in our gastrointestinal tracts, more than 10 times that of the total number of cells in human bodies. They consist of more than 1000 species, and contain 150 times as many genes as our genomes. Colonizing soon after our birth, these microbes comprise a huge community and closely interact with their hosts, having great influence on our '''immune systems''', '''endocrine''', '''metabolic states''', and even '''nervous systems''', from birth to death, from health to illness. They seem to be tiny natural machines that can be utilized to carry the blueprint of our design and function adaptively and communicably inside our bodies. Weaving into the fabric of the complexity and adaptability of these intestinal microbial communities, we can achieve desirable medical goals, in our case, production and delivery of peptide drugs. Operation and customization of these '''complex adaptive biosystems''' will be an inevitable trend towards the development of systems biology and synthetic bio-techniques. |
==Peptide-Based Therapies== | ==Peptide-Based Therapies== | ||
Revision as of 17:29, 26 October 2012
Background
Contents |
Complex Adaptive BioSystems
Human bodies are highly fluctuating complex systems. They detect and integrate the clues from changing environments and their own internal states, making numerous responses after delicate computation and regulation. Traditional routes of drug administration including oral intake and intravenous injection may be too simplified to promptly fit the real-time condition of the body states. In addition, the frequent and repetitive intake of drugs may be annoying, and sometimes the invasive processes are afflictive, bringing inconvenience to our daily lives. Medical instruments or electrical monitors can instantaneously detect and response to some specific physiological or pathological parameters, but they are usually too heavy and bulky to carry, which restrict the mobility of patients while using it. Therefore we aim to program the intestinal microbes to build our novel smart drug delivery systems—PEPDEX.
There are around \(10^{13}\) to \(10^{14}\) microorganisms inhabiting in our gastrointestinal tracts, more than 10 times that of the total number of cells in human bodies. They consist of more than 1000 species, and contain 150 times as many genes as our genomes. Colonizing soon after our birth, these microbes comprise a huge community and closely interact with their hosts, having great influence on our immune systems, endocrine, metabolic states, and even nervous systems, from birth to death, from health to illness. They seem to be tiny natural machines that can be utilized to carry the blueprint of our design and function adaptively and communicably inside our bodies. Weaving into the fabric of the complexity and adaptability of these intestinal microbial communities, we can achieve desirable medical goals, in our case, production and delivery of peptide drugs. Operation and customization of these complex adaptive biosystems will be an inevitable trend towards the development of systems biology and synthetic bio-techniques.
Peptide-Based Therapies
We selected the peptide, which is specifically designed to fit MHC, which binds the processed antigens and presents them to T-cell. The variants of MHC from person to person result in different reaction once challenged by allergen, as the symptoms can be mild or more severe. This also points out one of the biggest issues in immune therapy-customized medications. We cannot treat the whole population with the same regimen. Many the advent of synthetic biology, bacteria is a potent carrier for short peptide when introduced to the gut, where it can easily colonize and be exposed to antigen presenting cells after phagocytosis by M-cell, the nature immune organ in intestine.
- Anti-allergy- Asthma (John D. Campbell, JEM, 2009) (Campbell, Buckland et al. 2009)
There are many mucosal-associated lymphoid systems in the human body, which can react quickly as they usually lie in the host-environmental interfaces. There are many site-specific name for these immune organs; for instance, they are called gut-associated lymphoid tissue system (GALT). In our delivery system, we aim to send and present our peptides through gut-associated lymphoid tissue, since it is one of the biggest and most sophisticated immune systems inside our body, and the system works pretty well in discriminating the pathogens and common normal flora. We try to manipulate this characteristic to design our anti-allergy peptide.
Microfold cells (Wershil and Furuta 2008), abbreviated M cell, are cells found in the follicle-associated epithelium, especially in guts, and deliver foreign antigen to the underlying dendritic cells. The interaction of dendritic cells with the different types of cognate T-cell decides the outcome of immune responses, either adaptive immune responses against certain pathogens or tolerance establishment. The main determinant of the two polarized responses is not yet clear, but the current assumption points to the different types of T-cells activated after antigenic challenges (Iwasaki and Kelsall 2001). In our project, we planned to deliver anti-allergic peptide by engineered E. coli, which would carries Fel d1, the major cat allergen, as documented(Campbell, Buckland et al. 2009). The anti-allergic peptide requires repetitive challenges, and thus express the Fel d1 through a constitutive, thermal-inducible promoter, phs (Taylor, Straus et al. 1984).
- Campbell, J. D., K. F. Buckland, et al. (2009). "Peptide immunotherapy in allergic asthma generates IL-10-dependent immunological tolerance associated with linked epitope suppression." J Exp Med 206(7): 1535-1547.
- Iwasaki, A. and B. L. Kelsall (2001). "Unique functions of CD11b+, CD8 alpha+, and double-negative Peyer's patch dendritic cells." J Immunol 166(8): 4884-4890.
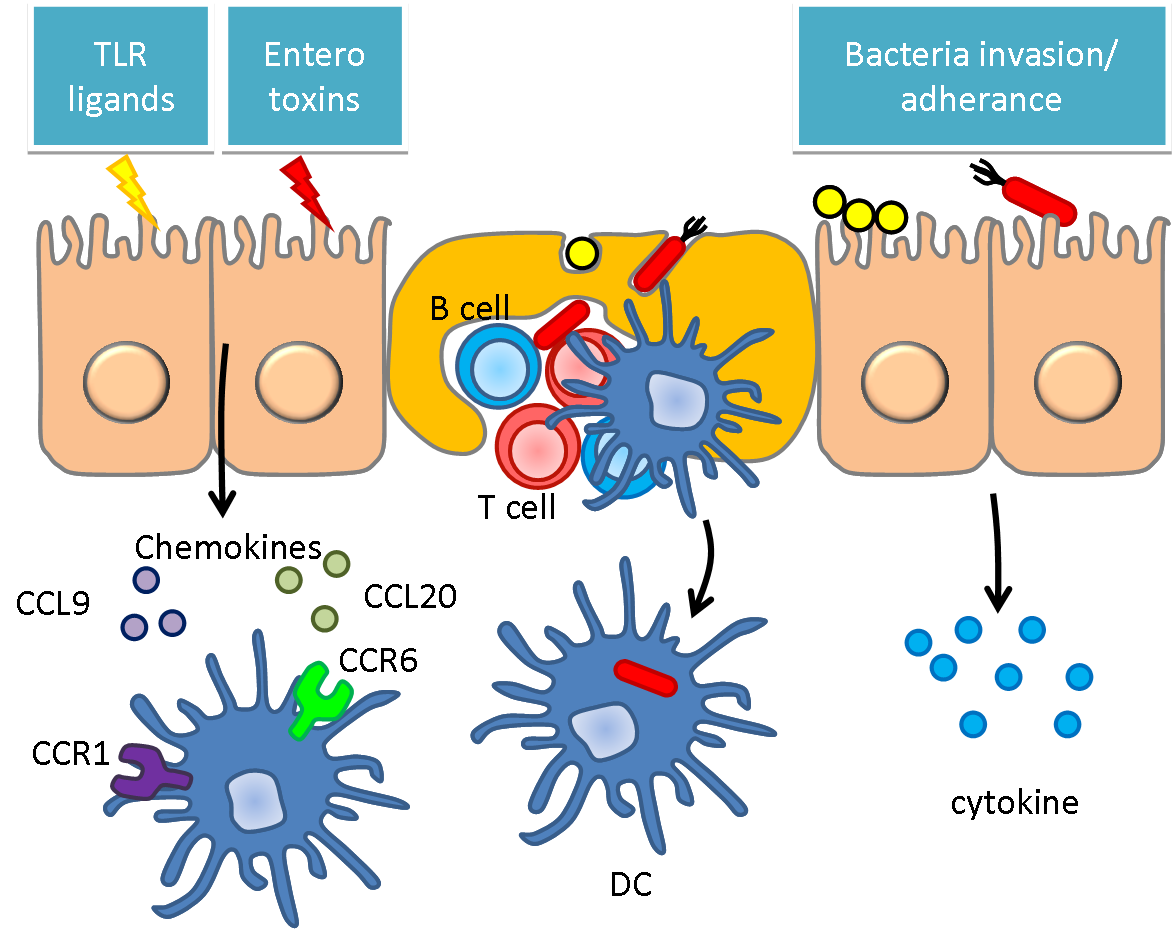
Bacteria and Immune System
Bacteria and Nervous System
The novel conceptual model “Brain-Gut Axis” has received emerging attention recently. Lots of studies showing evidence that intestinal microbiota have profound impacts on our nervous systems blossom during this 5~10 years. They interact with each other bidirectionally via various pathways. These include neuroendocrine (hypothalamus-pituitary-adrenal axis), immune systems (neuromodulating cytokines), enteric nervous systems and autonomic nervous systems (vagus nerve). Gut microbes produce substance such as tryptophan-related metabolites kynurenic acid, short chain fatty acids, and neurometabolites GABA, noradrenalin, and dopamine that potentially target to and influence functions of our central nervous systems. In the process of neurodevelopment, they modulate the expression level of many critical genes, such as brain-derived neurotropic factor (BDNF), NMDA receptors or 5-HT receptors and communicate with brain regions like striatum, hippocampus, amygdale, hypothalamus, and cingulated gyrus. It has long been known that the colonization of gut flora is related to the stress response of the hosts, changing their states of anxiety and exploratory behavior. Diseases such as inflammatory bowel diseases (IBS) and multiple sclerosis (MS) are also documented to be associated with intestinal microorganisms. New focus has been greatly put on many neuropsychiatric diseases, for instance, autism spectrum disorders (ASD), depression, anxiety disorders, and schizophrenia.
Reference
- Collins SM, et al. (2012) The interplay between the intestinal microbiota and the brain. Nature Reviews Microbiology. AOP, published online 24 September 2012, 1-8.
- Cryan JF, et al. (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. NatureReviews Neuroscience 13: 701-712.
- Rhee SH, et al. (2009) Principles and clinical implications of the brain–gut–enteric microbiota axis. Nature Rev. Gastroenterol. Hepatol. 6, 306–314.
- Neufeld KM, et al. (2010) Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 23, 255–264.
- Bercik, P. et al. (2011) The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141, 599–609.
- Lyte M, et al. (2006) Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol. Behav. 89, 350–357.
- Bravo, J. A. et al. (2011) Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl Acad. Sci. USA 108, 16050–16055.
- Wall, R. et al. (2012)Contrasting effects of Bifidobacterium breve NCIMB 702258 and Bifidobacterium breve DPC 6330 on the composition of murine brain fatty acids and gut microbiota. Am. J. Clin. Nutr. 95, 1278–1287.
- Tillisch, K. et al. (2012) Modulation of the brain–gut axis after 4 week intervention with a probiotic fermented dairy product. Gastroenterology 142, S-115.
- Mayer EA. (2011) Gut feelings: the emerging biology of gut–brain communication. Nature Rev. Neurosci. 12, 453–466.
- Freestone PP, et al. (2008) Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol. 16, 55–64.
- Kaper JB, et al. (2005). Bacterial cell to cell signaling in the gastrointestinal tract. Infect. Immun. 73, 3197–3209.
- Neufeld KM, et al. (2011). Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 23, 255–264.
- Heijtz RD, et al. (2011) Normal gut microbiota modulates brain development and behavior. Proc. Natl Acad. Sci. USA 108, 3047–3052.
- Gareau MG, et al. (2011) Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60, 307–317.
- Desbonnet L, et al. (2010) Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 170, 1179–1188.
- Lyte M. (2011) Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays 33, 574–581.
- Derecki, N. C. et al. (2010) Regulation of learning and memory by meningeal immunity: a key role for IL 4. J. Exp. Med. 207, 1067–1080.
- Lyte M, et al. (2011) Stress at the intestinal surface: catecholamines and mucosa– bacteria interactions. Cell Tissue Res. 2431, 23–32..
- Lee Y K, et al. (2011) Proinflammatory T cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 108, 4615–4622.
- Berer, K. et al. (2011) Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541.
- Juárez I, et al. (2008) Ontogeny of altered dendritic morphology in the rat prefrontal cortex, hippocampus, and nucleus accumbens following cesarean delivery and birth anoxia. J. Comp. Neurol. 507, 1734–1747.
 "
"