Team:NTU-Taida/Project/Background
From 2012.igem.org
Background
Contents |
Complex Adaptive BioSystems
Human bodies are highly fluctuating complex systems. They detect and integrate the clues from changing environments and their own internal states, making numerous responses after delicate computation and regulation. Traditional routes of drug administration including oral intake and intravenous injection may be too simplified to promptly fit the real-time condition of the body states. In addition, the frequent and repetitive intake of drugs may be annoying, and sometimes the invasive processes are afflictive, bringing inconvenience to our daily lives. Medical instruments or electrical monitors can instantaneously detect and response to some specific physiological or pathological parameters, but they are usually too heavy and bulky to carry, which restrict the mobility of patients while using it. Therefore we aim to program the intestinal microbes to build our novel smart drug delivery systems—PEPDEX.
There are around \(10^{13}\) to \(10^{14}\) microorganisms inhabiting in our gastrointestinal tracts, more than 10 times that of the total number of cells in human bodies. They consist of more than 1000 species, and contain 150 times as many genes as our genomes. Colonizing soon after our birth, these microbes comprise a huge community and closely interact with their hosts, having great influence on our immune systems, endocrine, metabolic states, and even nervous systems, from birth to death, from health to illness. They seem to be tiny natural machines that can be utilized to carry the blueprint of our design and function adaptively and communicably inside our bodies. Weaving into the fabric of the complexity and adaptability of these intestinal microbial communities, we can achieve desirable medical goals, in our case, production and delivery of peptide drugs. Operation and customization of these complex adaptive biosystems will be an inevitable trend towards the development of systems biology and synthetic bio-techniques.
Peptide-Based Therapies
General Description: Advantages
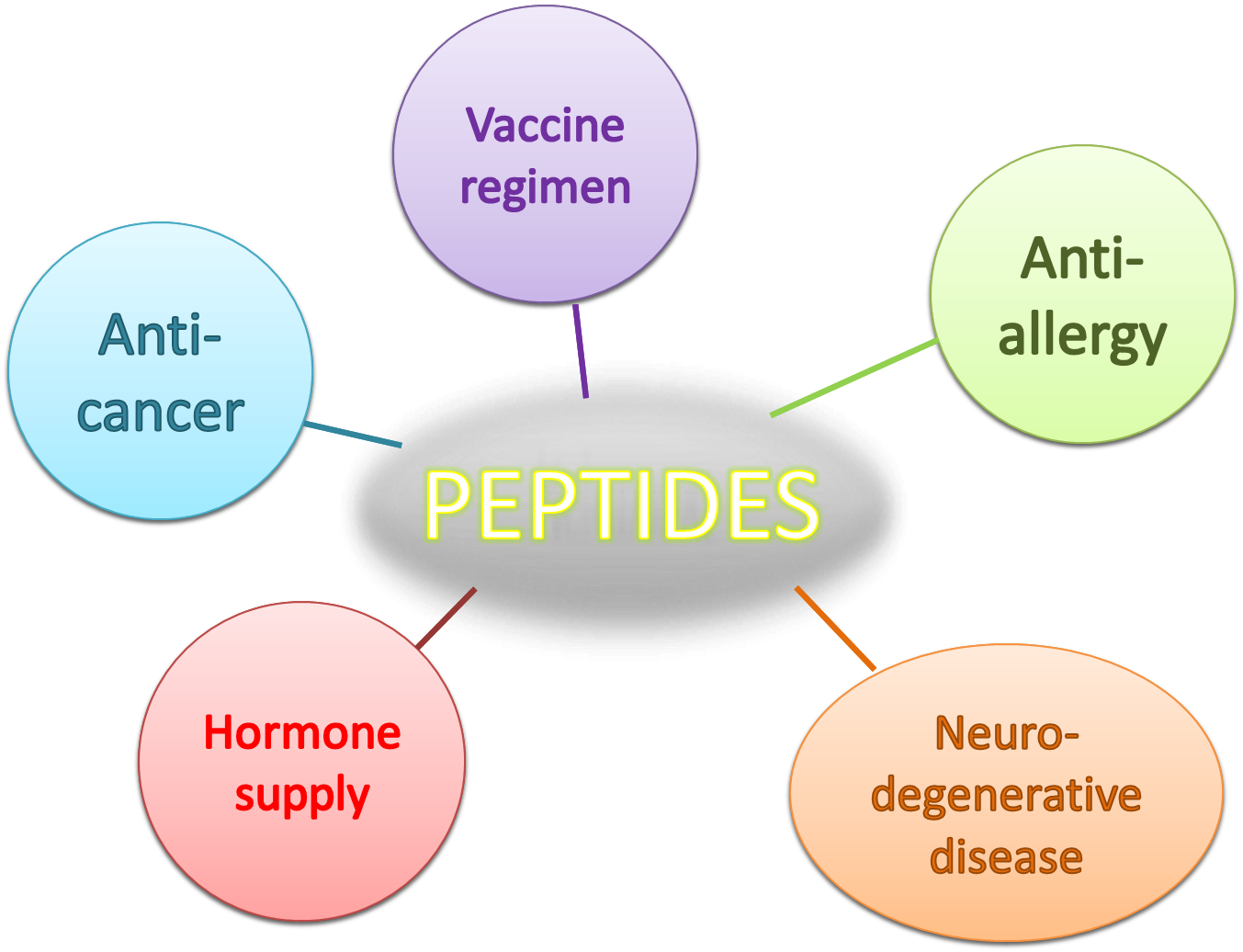
There has been a wide arrange of peptide-based drugs in the past one decades. Under the general physiological condition, peptides can be used as a hormone or paracrine to bridge the cell to cell or system to system communication. The peptide can be synthesized in a very short time, which acts as an ideal choice in quick responses to the outer stimulus. In addition, the peptides also play an important role in immune responses since many of the epitopes recognized by effector immune cells are short peptides. Many studies also prove the importance of certain amino acid sequences changes can lead to a huge difference in immune responses. And thus, based on this notion, peptide based vaccine is widely applied in many fields, for instance, anti-cancer vaccine and anti-allergy vaccine. In our project, PepDEX, we seek to deliver peptides which can also be quick synthesized by bacteria, and reach certain the therapeutic effects.
Applications in Immunology
When it comes to immunology, the peptide itself is ideal for different antigen design. We can easily select and engineer the peptide, which is specifically designed to fit MHC, which binds the processed antigens and presents them to T-cell. The variants of MHC from person to person result in different reaction once challenged by allergen, as the symptoms can be mild or more severe. This also points out one of the biggest issues in immune therapy-customized medications. We cannot treat the whole population with the same regimen. Many the advent of synthetic biology, bacteria is a potent carrier for short peptide when introduced to the gut, where it can easily colonize and be exposed to antigen presenting cells after phagocytosis by M-cell, the nature immune organ in intestine. The contact of immune cells to the bacteria provides the possibilities for inducing specific immune responses, which can be either reactive immune responses or senescence of the hypersensitivities of certain effector cells.
Applications in Neuroscience
Neuropeptides are short peptides that are secreted or act on the nervous systems. Receptors of neuropeptides are widely distributed in the brain and other parts of the nervous systems, such as diffuse modulating systems, HPA axis, autonomic nervous systems and enteric nervous systems (brain-gut axis). The actions of these neuropeptides broadly influence our emotion, stress response, pain, behavior, developmental and degenerative processes, mostly via signal pathway of G-protein coupled receptors. Agonists and antagonists of these neuropeptides receptors are practical therapuetics for many neuropsychiatric diseases, according to their relatively high specificity and efficacy. For instance, neuropeptide Y (NPY), substance P (SP) and corticotropin-releasing factor (CRF) have anxiolytic and psychotropic effects and can be used in anti-anxiety and anti-depression. Neurodegenerative diseases are also hot targets of peptide-based therapies. The glucagon-like peptide-1 (GLP-1), which we use as a demonstration in our project, has neuroprotective role of higher cortical functions via PI3K, PKA, and ERK pathway, and serves as a prevention for in Alzheimer’s disease and Parkinson’s disease due to its interaction with β-amyloid and protection of dopaminergic neurons. Besides, it can increase the long-term potentiation (LTP) and synaptic plasticity in hippocampal regions, improving learning and memory. Recently, emerging studies on the synaptogenesis and memory-promoting effects of angiotensin IV-related peptides also reveal its potential anti-dementia use. The development of these peptide-based therapies therefore receives more and more attention in health and medicine regimes.
Bacteria and Immune System
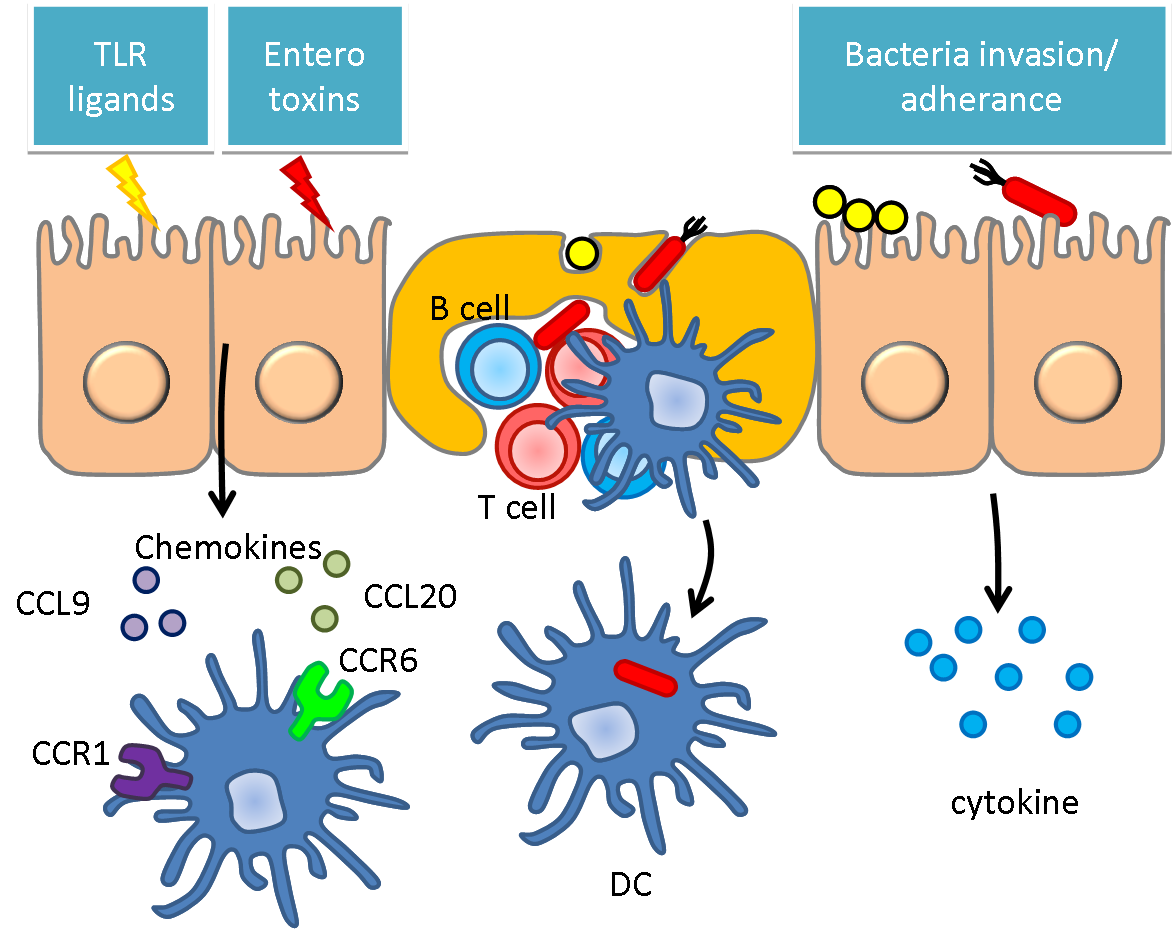
Human microbiome is an aggregation of microorganisms that resided on the surface and in the deep layer of skin. Say in intestine, there are over 100,000 primarily aerobic organisms per milliliter found. In intestine, one of the most pulsating and hectic environment over human body, lies over a trillion of cells, most of them bacteria. They are mostly composed of gram-positive Streptococcus, Staphylococcus, Lactobacillus, and gram-negative Bacillus.
In some of the particular cases, those bugs have some interaction with human body by manipulating the epithelial cells over small intestine. Instead of causing illness, those cells in the hosts in the gut tend to train our immune systems rather than spark an immune response. We can easily imagine a peculiar balance between the bacteria in the intestine and the host immunity. Our notion is pretty simple, if we can engineer the normal flora, there will be a huge amount of bacteria now working for us. In reverse, if we change some characteristic of the host microbiota, they may also induce certain amount of inflammation, which involves adaptive immune systems, and this opens up the possibilities for delivering certain anti-infectious and anti-cancer peptides to reach significant therapeutic effects.
Bacteria and Nervous System
The novel conceptual model “Brain-Gut-Microbiota Axis” has received emerging attention recently. Lots of studies showing evidence that intestinal microbiota have profound impacts on our nervous systems blossom during this 5~10 years. They interact with each other bidirectionally via various pathways. These include neuroendocrine (hypothalamus-pituitary-adrenal axis), immune systems (neuromodulating cytokines), enteric nervous systems and autonomic nervous systems (vagus nerve). Gut microbes produce substance such as tryptophan-related metabolites kynurenic acid, short chain fatty acids, and neurometabolites GABA, noradrenalin, and dopamine that potentially target to and influence functions of our central nervous systems. In the process of neurodevelopment, they modulate the expression level of many critical genes, such as brain-derived neurotropic factor (BDNF), NMDA receptors or 5-HT receptors and communicate with brain regions like striatum, hippocampus, amygdale, hypothalamus, and cingulated gyrus. It has long been known that the colonization of gut flora is related to the stress response of the hosts, changing their states of anxiety and exploratory behavior. Diseases such as inflammatory bowel diseases (IBS) and multiple sclerosis (MS) are also documented to be associated with intestinal microorganisms. New focus has been greatly put on many neuropsychiatric diseases, for instance, autism spectrum disorders (ASD), depression, anxiety disorders, and schizophrenia.
Reference
- Collins SM, et al. (2012) The interplay between the intestinal microbiota and the brain. Nature Reviews Microbiology. AOP, published online 24 September 2012, 1-8.
- Cryan JF, et al. (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. NatureReviews Neuroscience 13: 701-712.
- Rhee SH, et al. (2009) Principles and clinical implications of the brain–gut–enteric microbiota axis. Nature Rev. Gastroenterol. Hepatol. 6, 306–314.
- Neufeld KM, et al. (2010) Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 23, 255–264.
- Bercik, P. et al. (2011) The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141, 599–609.
- Lyte M, et al. (2006) Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol. Behav. 89, 350–357.
- Bravo, J. A. et al. (2011) Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl Acad. Sci. USA 108, 16050–16055.
- Wall, R. et al. (2012)Contrasting effects of Bifidobacterium breve NCIMB 702258 and Bifidobacterium breve DPC 6330 on the composition of murine brain fatty acids and gut microbiota. Am. J. Clin. Nutr. 95, 1278–1287.
- Tillisch, K. et al. (2012) Modulation of the brain–gut axis after 4 week intervention with a probiotic fermented dairy product. Gastroenterology 142, S-115.
- Mayer EA. (2011) Gut feelings: the emerging biology of gut–brain communication. Nature Rev. Neurosci. 12, 453–466.
- Freestone PP, et al. (2008) Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol. 16, 55–64.
- Kaper JB, et al. (2005). Bacterial cell to cell signaling in the gastrointestinal tract. Infect. Immun. 73, 3197–3209.
- Neufeld KM, et al. (2011). Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 23, 255–264.
- Heijtz RD, et al. (2011) Normal gut microbiota modulates brain development and behavior. Proc. Natl Acad. Sci. USA 108, 3047–3052.
- Gareau MG, et al. (2011) Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60, 307–317.
- Desbonnet L, et al. (2010) Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 170, 1179–1188.
- Lyte M. (2011) Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays 33, 574–581.
- Derecki, N. C. et al. (2010) Regulation of learning and memory by meningeal immunity: a key role for IL 4. J. Exp. Med. 207, 1067–1080.
- Lyte M, et al. (2011) Stress at the intestinal surface: catecholamines and mucosa– bacteria interactions. Cell Tissue Res. 2431, 23–32..
- Lee Y K, et al. (2011) Proinflammatory T cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 108, 4615–4622.
- Berer, K. et al. (2011) Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541.
- Juárez I, et al. (2008) Ontogeny of altered dendritic morphology in the rat prefrontal cortex, hippocampus, and nucleus accumbens following cesarean delivery and birth anoxia. J. Comp. Neurol. 507, 1734–1747.
 "
"