Team:NTU-Taida/Project/Safety
From 2012.igem.org
(→Safety of Delivery System) |
(→Safety of Delivery System) |
||
| Line 8: | Line 8: | ||
[[File:NTU-Taida-Suicide-system.png|300px|thumb|left|alt=new idea about safety|general scheme of suicide system ]] | [[File:NTU-Taida-Suicide-system.png|300px|thumb|left|alt=new idea about safety|general scheme of suicide system ]] | ||
| - | <p style="text-indent: 2em;">However, these designs cannot completely eliminate the risk of horizontal gene transfer (HGT), where recombinant genes can move to other organisms independent of suicide system. Therefore, in addition to suicide system, we propose a new concept to deal with HGT risks by RNA interaction[[#Ref1|[1] ]]. A recent study has demonstrated that expressing peptide nucleic acid (PNA) antisense to antitoxin RNA 5' sequence kills bacteria cells [[#Ref2|[2] ]]. We reason that adding rationally designed antisense RNAs on the untranslated region of transcripts may interfere with target RNA’s function or translation, and we can employ this property to prevent HGT. For instance, HGT is more likely to occur between related species, such as lab E. coli & O157. Laboratory E. coli have inactivated all its hok/sok toxin-antitoxin system by mutation, but wild bacteria (especially pathogenic bacteria) usually have more active TA locus on its chromosome like E. coli O157. We plan to put a stem loop from hok mRNA, which can pair with sok RNA, 5’sequence on UTR of antibiotic resistance genes used in our PepdEx system. IF wild bacteria steal our antibiotic resistance genes and express it, its antitoxin will be competitive inhibited, and its toxin will get expressed to kill the thief. This prevents HGT between lab & wild coli. This concept can have wide extension. In addition to targeting antitoxin (functional RNA) of type I TA, it is also possible to design antisense sequences that target RBS to down-regulate targeted protein. Targeting antitoxin of type II TA, essential genes for metabolism, housekeeping genes and any sequences exist in potential HGT receivers but NOT our coli can also be used. Even if the design cannot kill thieves, it can still weaken receivers and reduce the advantages a stolen antibiotic gene may bring in, thus reducing the degree of danger in HGT.</p> | + | <p style="text-indent: 2em;">However, these designs cannot completely eliminate the risk of horizontal gene transfer (HGT), where recombinant genes can move to other organisms independent of suicide system. Therefore, in addition to suicide system, we propose a new concept to deal with HGT risks by RNA interaction[[#Ref1|[1] ]]. Although bacteria lack RNAi pathway, expressing well designed antisense RNAs have been shown to have inhibitory effect on target RNAs through competitive inhibition.A recent study has demonstrated that expressing peptide nucleic acid (PNA) antisense to antitoxin RNA 5' sequence kills bacteria cells [[#Ref2|[2] ]]. We reason that adding rationally designed antisense RNAs on the untranslated region of transcripts may interfere with target RNA’s function or translation, and we can employ this property to prevent HGT. For instance, HGT is more likely to occur between related species, such as lab E. coli & O157. Laboratory E. coli have inactivated all its hok/sok toxin-antitoxin system by mutation, but wild bacteria (especially pathogenic bacteria) usually have more active TA locus on its chromosome like E. coli O157. We plan to put a stem loop from hok mRNA, which can pair with sok RNA, 5’sequence on UTR of antibiotic resistance genes used in our PepdEx system. IF wild bacteria steal our antibiotic resistance genes and express it, its antitoxin will be competitive inhibited, and its toxin will get expressed to kill the thief. This prevents HGT between lab & wild coli. This concept can have wide extension. In addition to targeting antitoxin (functional RNA) of type I TA, it is also possible to design antisense sequences that target RBS to down-regulate targeted protein. Targeting antitoxin of type II TA, essential genes for metabolism, housekeeping genes and any sequences exist in potential HGT receivers but NOT our coli can also be used. Even if the design cannot kill thieves, it can still weaken receivers and reduce the advantages a stolen antibiotic gene may bring in, thus reducing the degree of danger in HGT.</p> |
<p style="text-indent: 2em;">A final note: in the past, the repression efficiencies of antisense RNA in bacteria are low. However, after the invention of paired termini antisense RNA (PTasRNA) method, incorporation of U turn/YUNR motif, etc., the concept of competitive inhibition will become more and more feasible for the microbial synthetic biology community[[#Ref1|[1 , ]][[#Ref3|3 , ]][[#Ref4|4] ]].</p> | <p style="text-indent: 2em;">A final note: in the past, the repression efficiencies of antisense RNA in bacteria are low. However, after the invention of paired termini antisense RNA (PTasRNA) method, incorporation of U turn/YUNR motif, etc., the concept of competitive inhibition will become more and more feasible for the microbial synthetic biology community[[#Ref1|[1 , ]][[#Ref3|3 , ]][[#Ref4|4] ]].</p> | ||
Revision as of 03:55, 27 September 2012
Safety
Safety of Delivery System
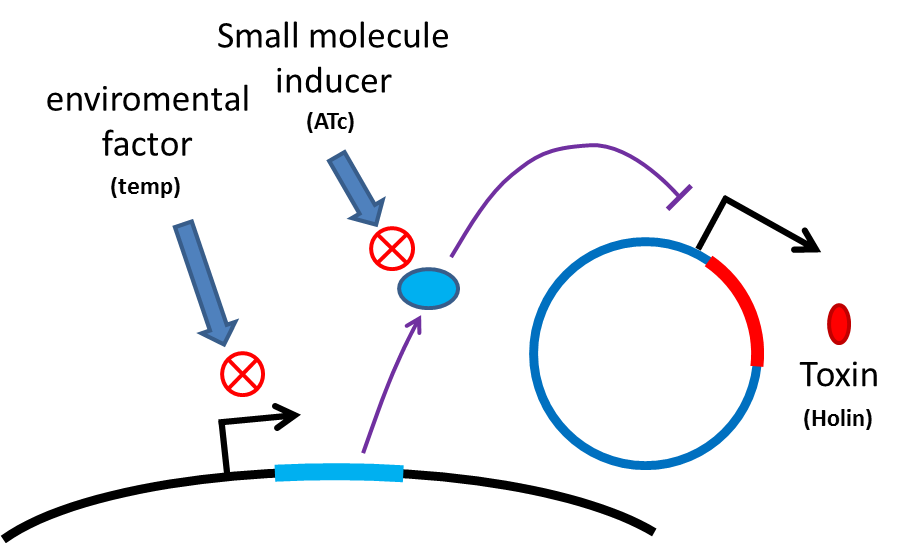
Many turn off strategies have been developed, most of them are inducible suicide systems that can be activated at certain conditions. In our project, we plan to use temperature as the activating signal (or alternatively, small molecules). When the course of treatment ends, leaving human body (temperature drop from 37C to room temperature) or administration of small amount of drugs (e.g. tetracycline) will cause suicide gene activation, thereby avoiding recombinant strain/gene pollution. And splitting suicide system to provide repression in trans can prevent plasmid transferring to wild type strains. Many off-the-rack parts can be used.
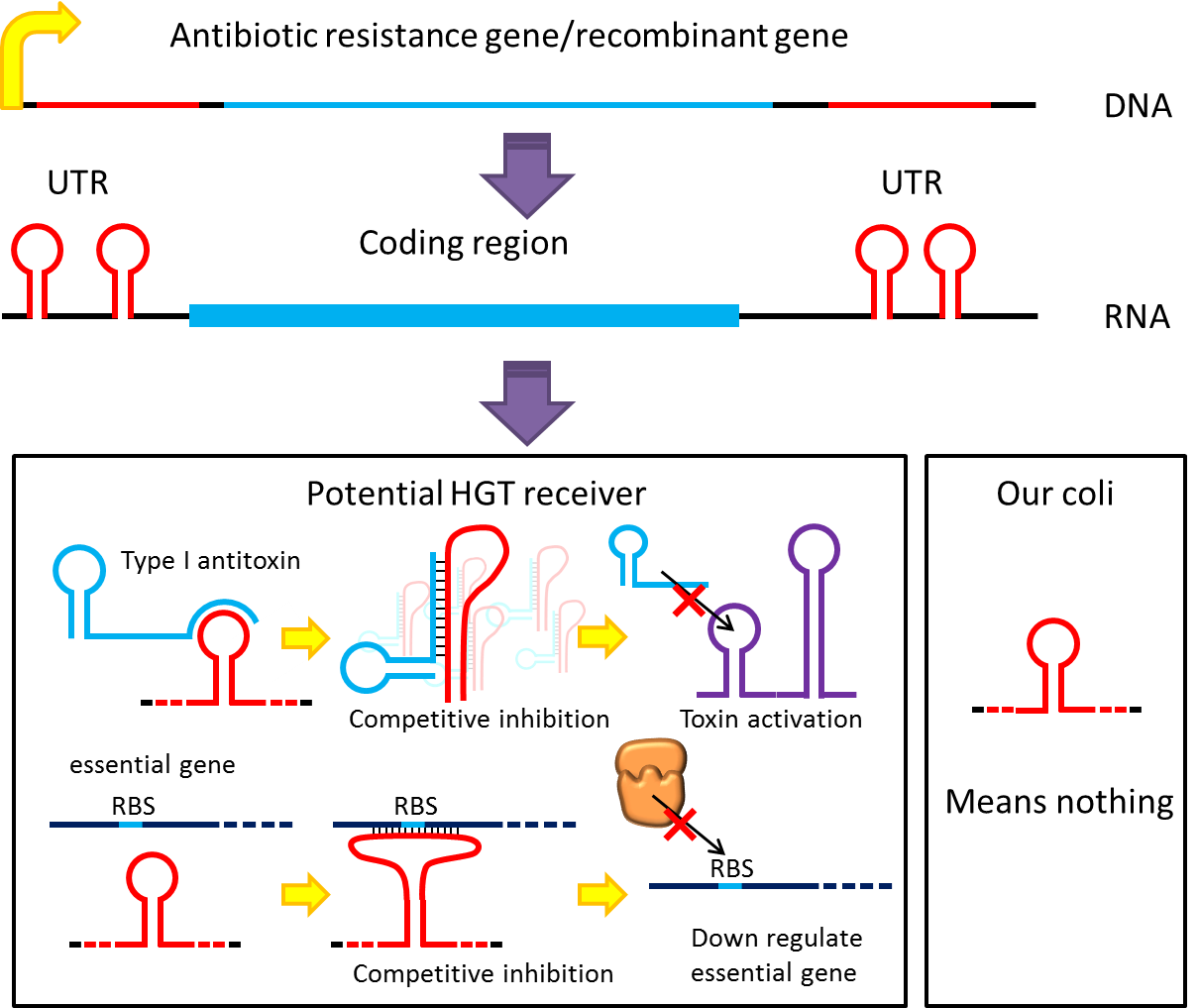
However, these designs cannot completely eliminate the risk of horizontal gene transfer (HGT), where recombinant genes can move to other organisms independent of suicide system. Therefore, in addition to suicide system, we propose a new concept to deal with HGT risks by RNA interaction[1] . Although bacteria lack RNAi pathway, expressing well designed antisense RNAs have been shown to have inhibitory effect on target RNAs through competitive inhibition.A recent study has demonstrated that expressing peptide nucleic acid (PNA) antisense to antitoxin RNA 5' sequence kills bacteria cells [2] . We reason that adding rationally designed antisense RNAs on the untranslated region of transcripts may interfere with target RNA’s function or translation, and we can employ this property to prevent HGT. For instance, HGT is more likely to occur between related species, such as lab E. coli & O157. Laboratory E. coli have inactivated all its hok/sok toxin-antitoxin system by mutation, but wild bacteria (especially pathogenic bacteria) usually have more active TA locus on its chromosome like E. coli O157. We plan to put a stem loop from hok mRNA, which can pair with sok RNA, 5’sequence on UTR of antibiotic resistance genes used in our PepdEx system. IF wild bacteria steal our antibiotic resistance genes and express it, its antitoxin will be competitive inhibited, and its toxin will get expressed to kill the thief. This prevents HGT between lab & wild coli. This concept can have wide extension. In addition to targeting antitoxin (functional RNA) of type I TA, it is also possible to design antisense sequences that target RBS to down-regulate targeted protein. Targeting antitoxin of type II TA, essential genes for metabolism, housekeeping genes and any sequences exist in potential HGT receivers but NOT our coli can also be used. Even if the design cannot kill thieves, it can still weaken receivers and reduce the advantages a stolen antibiotic gene may bring in, thus reducing the degree of danger in HGT.
A final note: in the past, the repression efficiencies of antisense RNA in bacteria are low. However, after the invention of paired termini antisense RNA (PTasRNA) method, incorporation of U turn/YUNR motif, etc., the concept of competitive inhibition will become more and more feasible for the microbial synthetic biology community[1 , 3 , 4] .
Reference
- Good, L. and J.E. Stach, Synthetic RNA silencing in bacteria - antimicrobial discovery and resistance breaking. Front Microbiol, 2011. 2: p. 185.
- Faridani, O.R., et al., Competitive inhibition of natural antisense Sok-RNA interactions activates Hok-mediated cell killing in Escherichia coli. Nucleic Acids Research, 2006. 34(20): p. 5915-22.
- Lucks, J.B., et al., Versatile RNA-sensing transcriptional regulators for engineering genetic networks. Proc Natl Acad Sci U S A, 2011. 108(21): p. 8617-22.
- Franch, T., et al., Antisense RNA regulation in prokaryotes: rapid RNA/RNA interaction facilitated by a general U-turn loop structure. Journal of Molecular Biology, 1999. 294(5): p. 1115-25.
 "
"