Team:Bielefeld-Germany/Results/substrate
From 2012.igem.org
(→Outlook) |
(→Dilution series) |
||
| Line 51: | Line 51: | ||
==Dilution series== | ==Dilution series== | ||
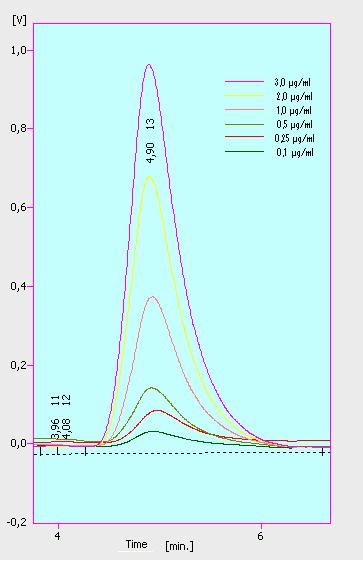
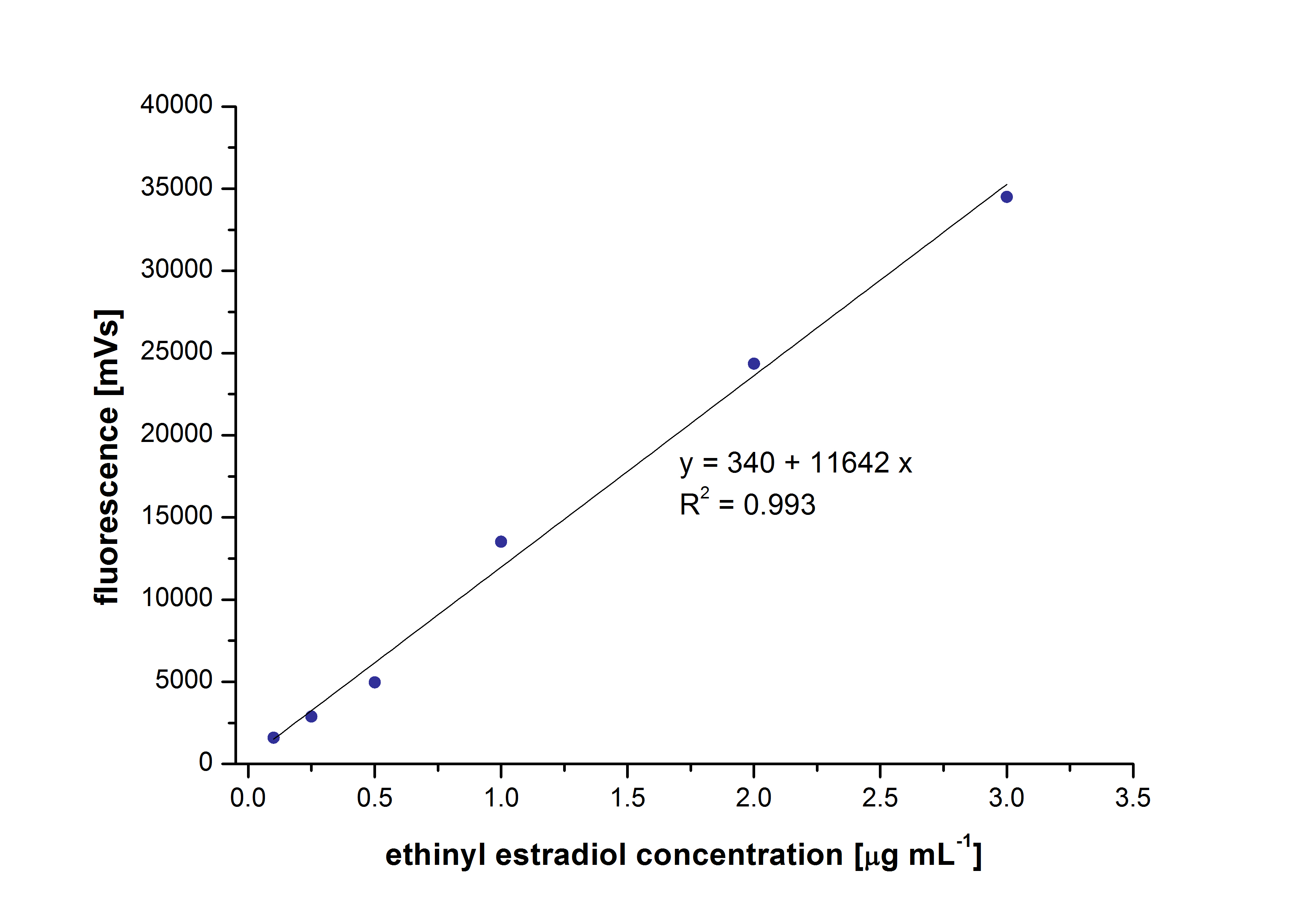
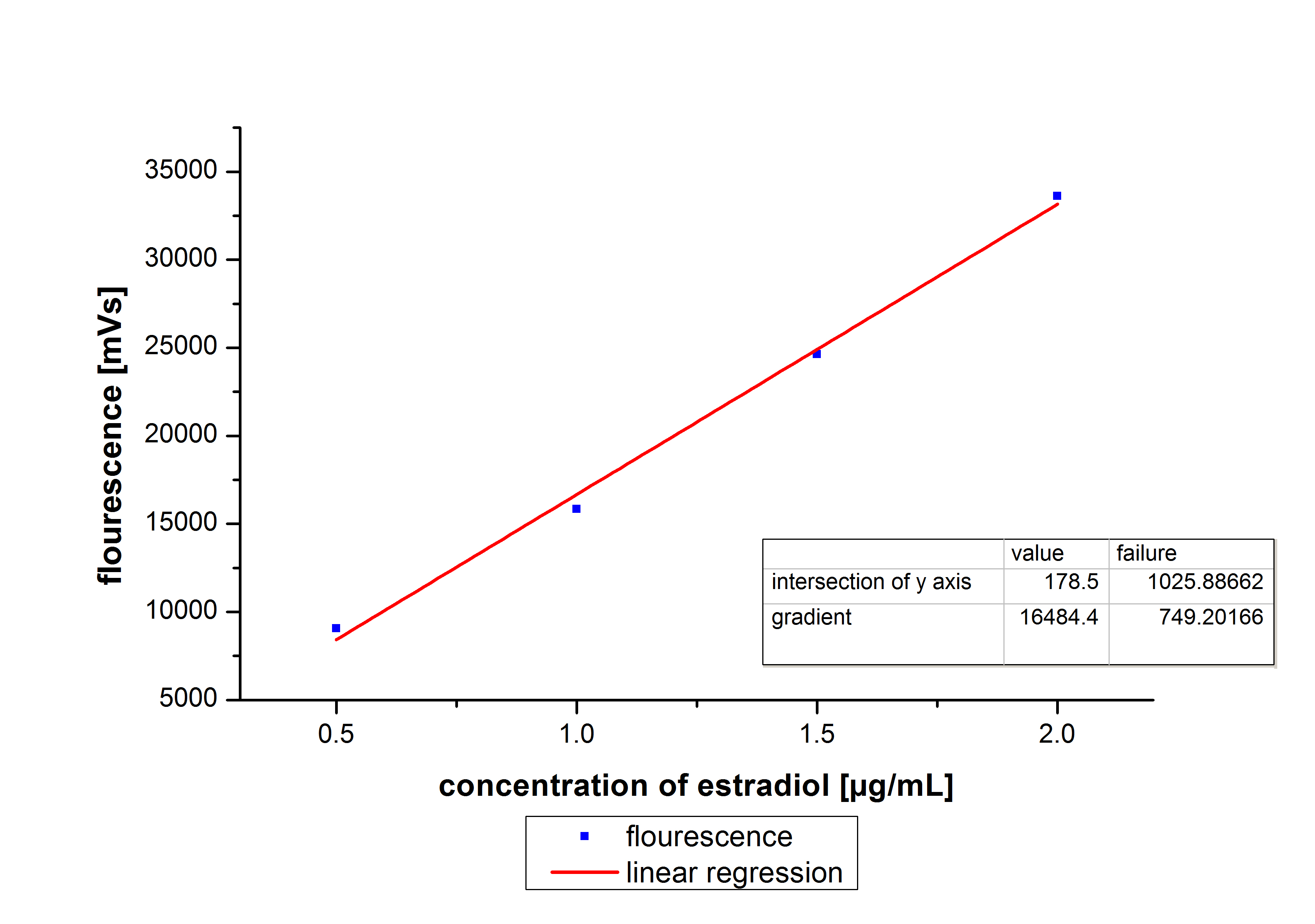
[[File:Bielefeld2012_detectionlimit.JPG|200px|thumb|right|'''Figure 1.1:'''The detection range of ethinyl estradiol. Concentrations between 0.1 µg ml <sup>-1</sup> and 3 µg ml <sup>-1</sup> a/partinfo> in a Bioengineering NFL22 with a total volume of 6re detecable.]] | [[File:Bielefeld2012_detectionlimit.JPG|200px|thumb|right|'''Figure 1.1:'''The detection range of ethinyl estradiol. Concentrations between 0.1 µg ml <sup>-1</sup> and 3 µg ml <sup>-1</sup> a/partinfo> in a Bioengineering NFL22 with a total volume of 6re detecable.]] | ||
| - | + | At first we measured the dilution series of all different substrates. While we were able to measure estradiol and ethinyl estradiol ,we did not succeed with the estrone calibration curves. This was caused by its bad solubility. The retention time for estradiol is 5.8 minutes, for estrone 4.7 minutes and for ethinyl estradiol 5.2 minutes. For all estrogens we could use the same extinction and emission values: Ex 230, Em 310. | |
| - | At first we | + | |
| - | + | ||
<br style="clear: both" /> | <br style="clear: both" /> | ||
| Line 62: | Line 60: | ||
<br style="clear: both" /> | <br style="clear: both" /> | ||
| - | The next substrate class | + | The next substrate class were the analgesics. These three substrates have different optimal extinction and emission values. Additional, difficulties occurred with naproxene and ibuprofen. Instead of one single peak we found two for each substrate, and none of them correlated with the used concentration. With diclofenac we are still not sure which extinction and emission values are to use. We found different values and additionaly we analysed it with a spectrofluorophotometer but this has also shown no clear peak for diclofenac. |
| - | Three of the four | + | Three of the four PAHs have the same extinction and emission values. Similar to the estrogens,the PAH calibration curves were generated. Naphthalene has a retention time of 9.6 minutes and its detection range is also 0.1 to 2.5 µg mL<sup>-1</sup>. Acenaphthene with a retention time of 15.1 minutes and phenantrene with a retention time of 17 minutes have maximal detectable concentration of 1.5 µg*mL<sup>-1</sup>.. |
Anthracene will be mesured later. | Anthracene will be mesured later. | ||
Revision as of 01:27, 27 September 2012

Summary
Contents |
Spectrofluorophotometer Anaylses
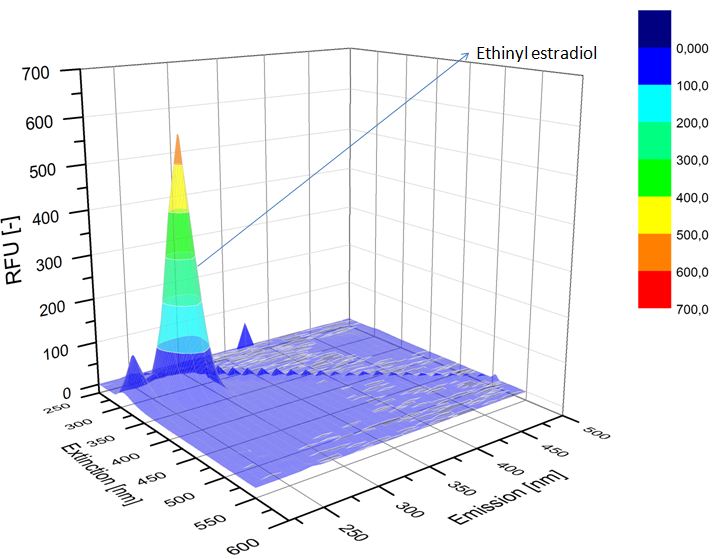
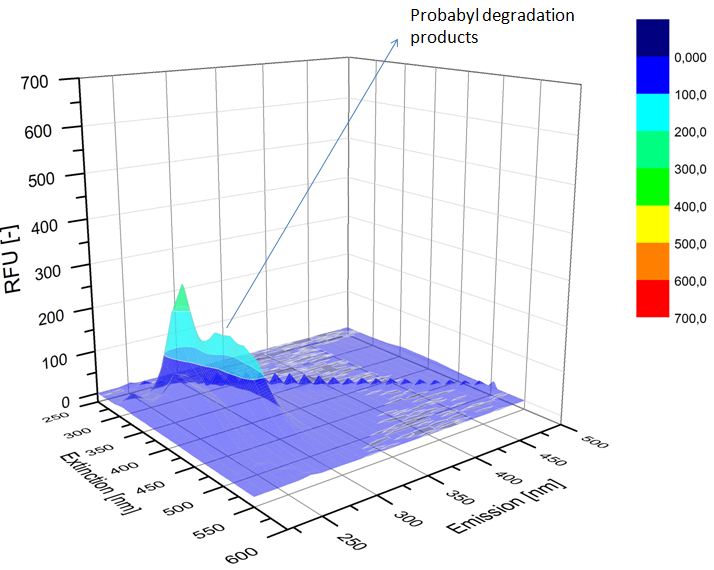
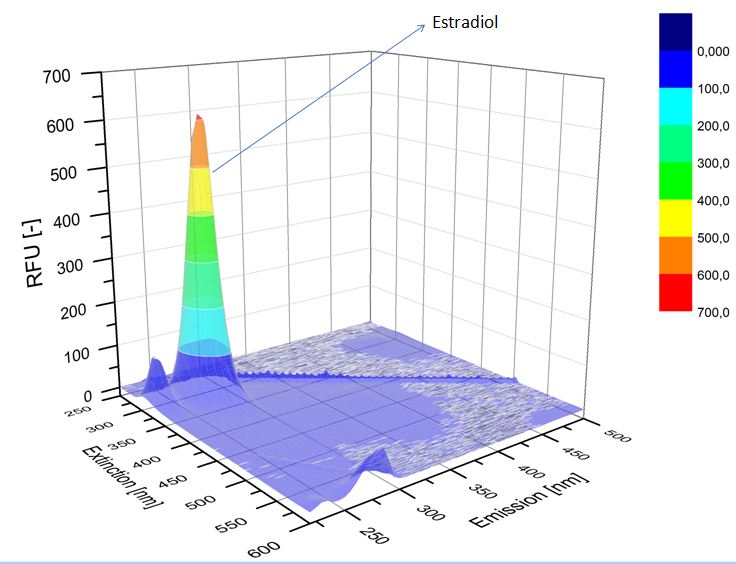
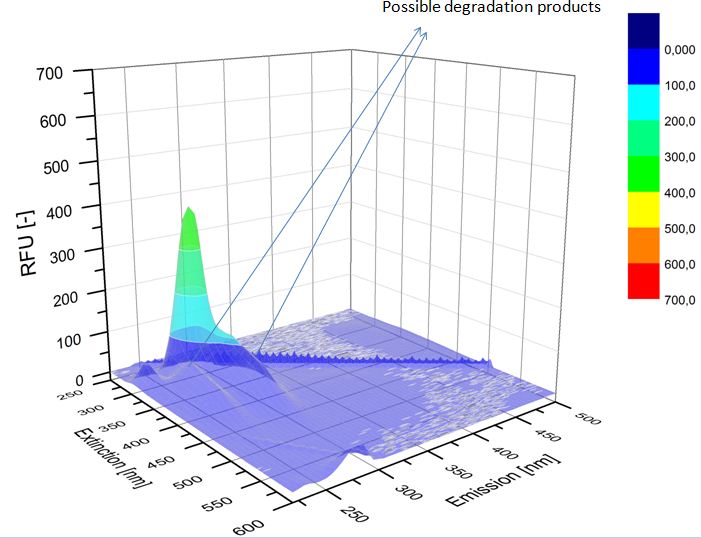
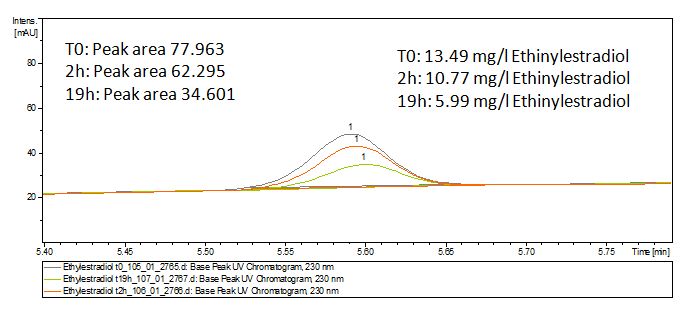
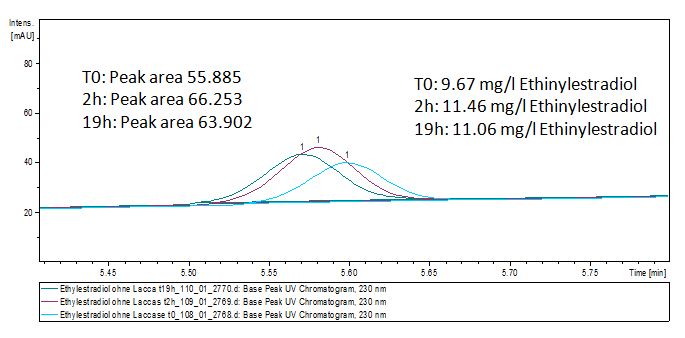
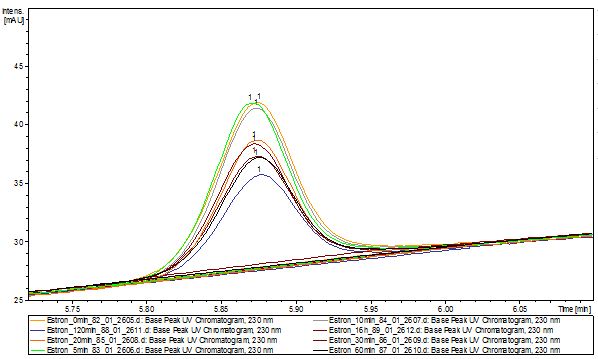
We analyzed the degradation of our subtrates with the Spectrofluorophotometer. As you can see it in the figures below the ethinyl estradiol and estradiol are degraded over night. Figure 1 shows the ethiny estradiol without laccase treatment, Figure 2 shows that no more ethinyl estradiol can be detected in the sample after the degradation and new peaks appear, , which might be represent possibly degradation products. In Figure 3 you can see the estradiol control without laccases. Like ethinyl estradiol our estradiol the peak does not appear after the degradation and new peaks appear indicating that thoose are new degradation products.
Liquid chromatography–mass spectrometry
Dilution series
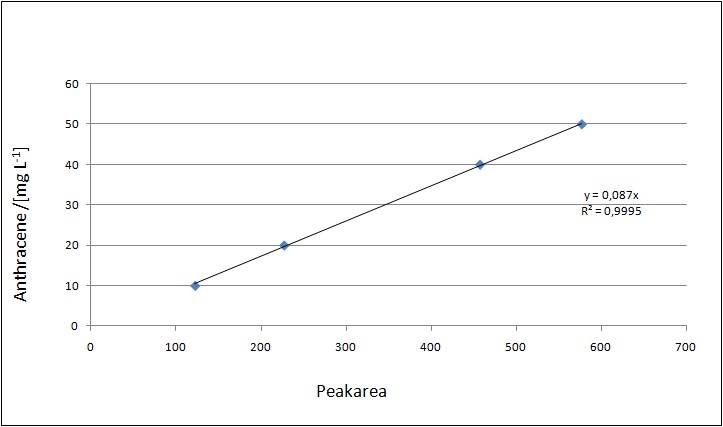
Our substrates are soluble in methanol. We set the standardts to a concentration of 1 mg mL-1. The upper detection limit for the LC-MS was evaluated at concentration of 10µg/l for the substrates esteron and estradiol. The same limit of detection was used for ethinyl estradiol and anthracen. We only used thoose four substrates. For all LC-MS sample preparations we used the T. versicolor laccases. The dilution series was prepared in methanol and 50% acetonitril-water (v/v).
Degradation results
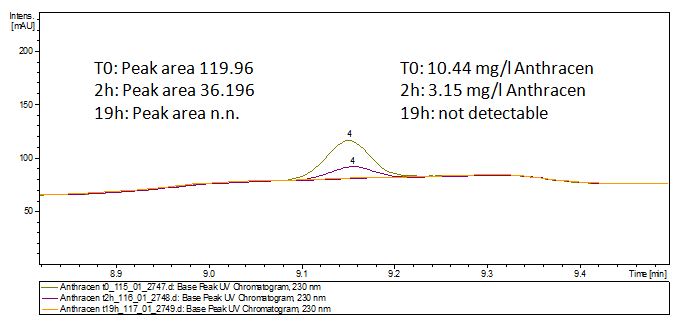
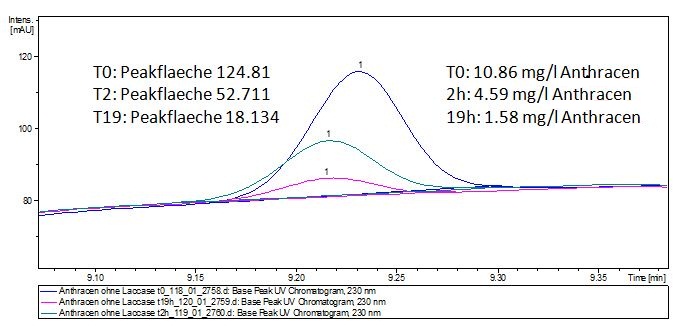
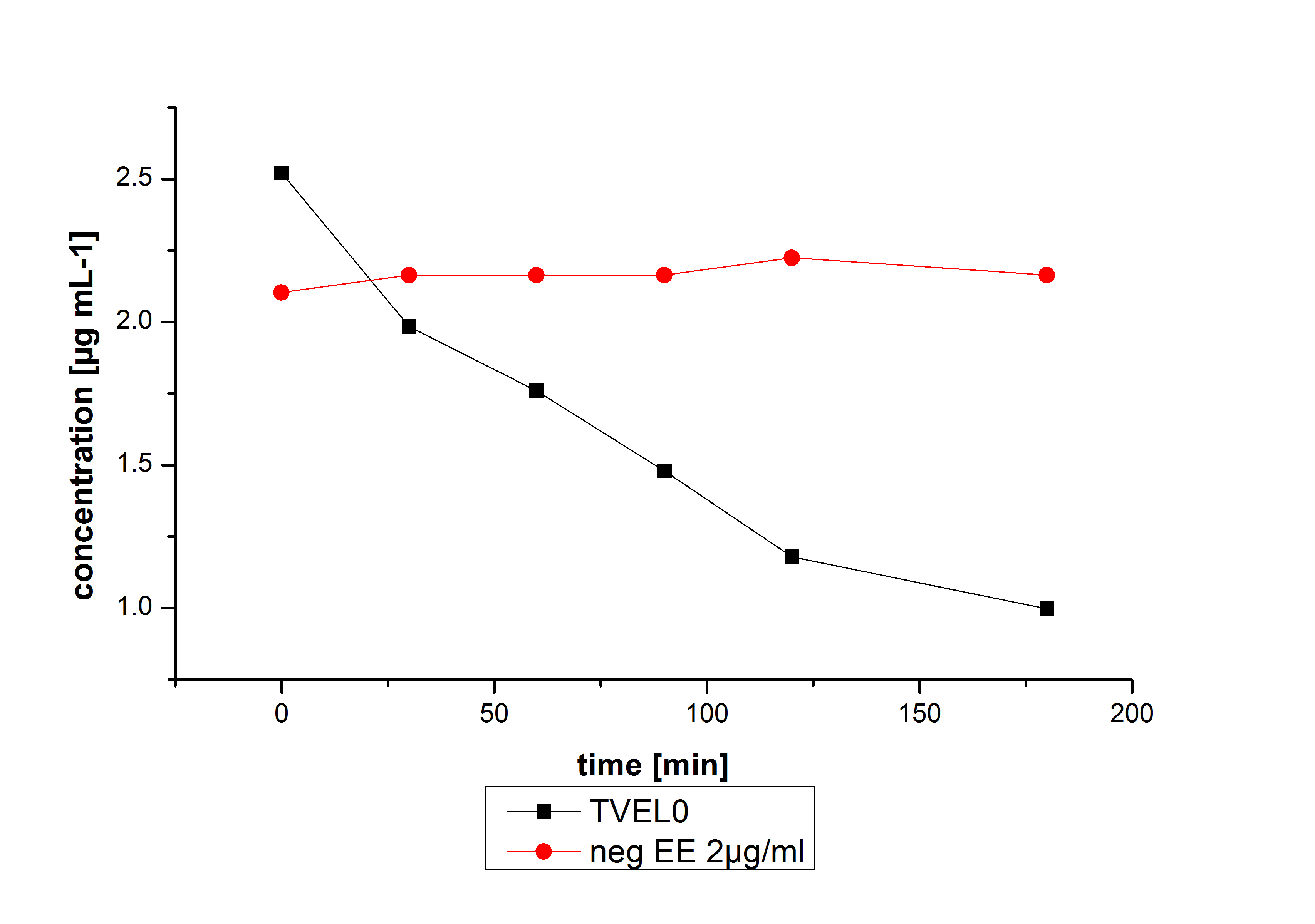
The TVEL0 was able to degrade the synthetic estradiol (fig. 1) and propably anthracen. (fig 3.). The ethinylestradiol calibration curve showed that it is stable in the used media (fig 2.). Anthracen disintegrates in the Britton Puffer. But it could be observed, that there is less anthracen measurable with the LC-MS. The results indicate, that thelaccase is able to degrade anthracene(fig. 4). Estrone (fig. 5) and estradiol (fig. 6) were degraded as well. Using estrone we could not identify any degradation products. The reason for this could be that the products are not detectable with LC-MS or with methods applied. We could identify peaks in the degradation of estradiol but were not able to identify them. In the following figures one can see the results of the LC-MS measurements. The controls in different media showed no changes in concentration.
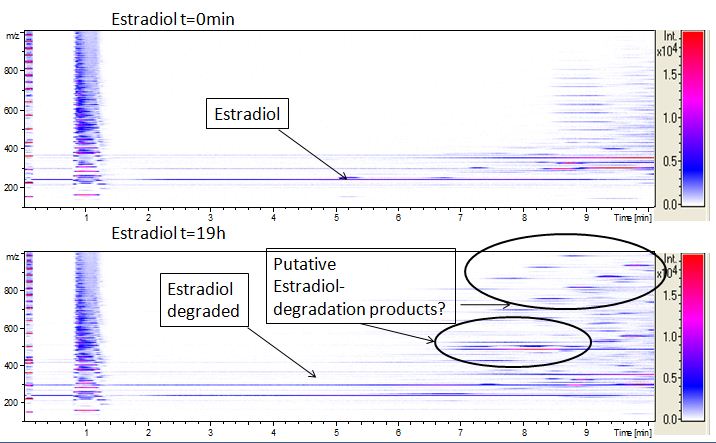
We also tried to measure the degradation using mass-spectrometry. Since quantification via mass-spectrometry is difficult regarding the ionization of the analytes, we quantified our substrates by UV-light. Nevertheless, mass spectrometry enables identification of possible degradation products. We anaylzed estradiol degradation in detail (fig. 6), resulting in the detection of possible chemical compounds generated during the (enzymatic) degradation. Until now we are not sure how estradiol degradation works, but with more time granted, the degradation products can be identified.
High performance liquid chromatography
Dilution series
At first we measured the dilution series of all different substrates. While we were able to measure estradiol and ethinyl estradiol ,we did not succeed with the estrone calibration curves. This was caused by its bad solubility. The retention time for estradiol is 5.8 minutes, for estrone 4.7 minutes and for ethinyl estradiol 5.2 minutes. For all estrogens we could use the same extinction and emission values: Ex 230, Em 310.
The next substrate class were the analgesics. These three substrates have different optimal extinction and emission values. Additional, difficulties occurred with naproxene and ibuprofen. Instead of one single peak we found two for each substrate, and none of them correlated with the used concentration. With diclofenac we are still not sure which extinction and emission values are to use. We found different values and additionaly we analysed it with a spectrofluorophotometer but this has also shown no clear peak for diclofenac.
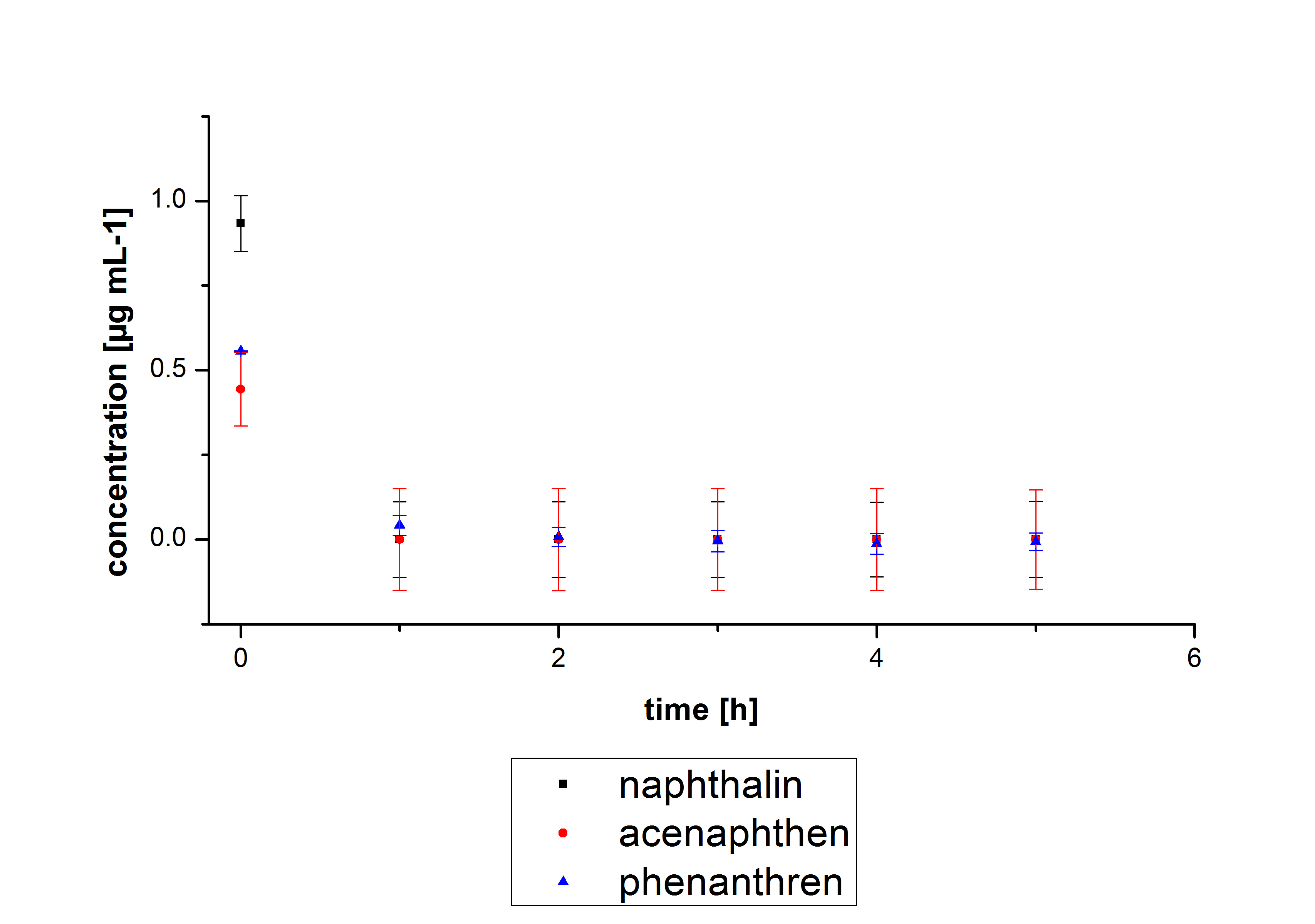
Three of the four PAHs have the same extinction and emission values. Similar to the estrogens,the PAH calibration curves were generated. Naphthalene has a retention time of 9.6 minutes and its detection range is also 0.1 to 2.5 µg mL-1. Acenaphthene with a retention time of 15.1 minutes and phenantrene with a retention time of 17 minutes have maximal detectable concentration of 1.5 µg*mL-1..
Anthracene will be mesured later.
Negative controls
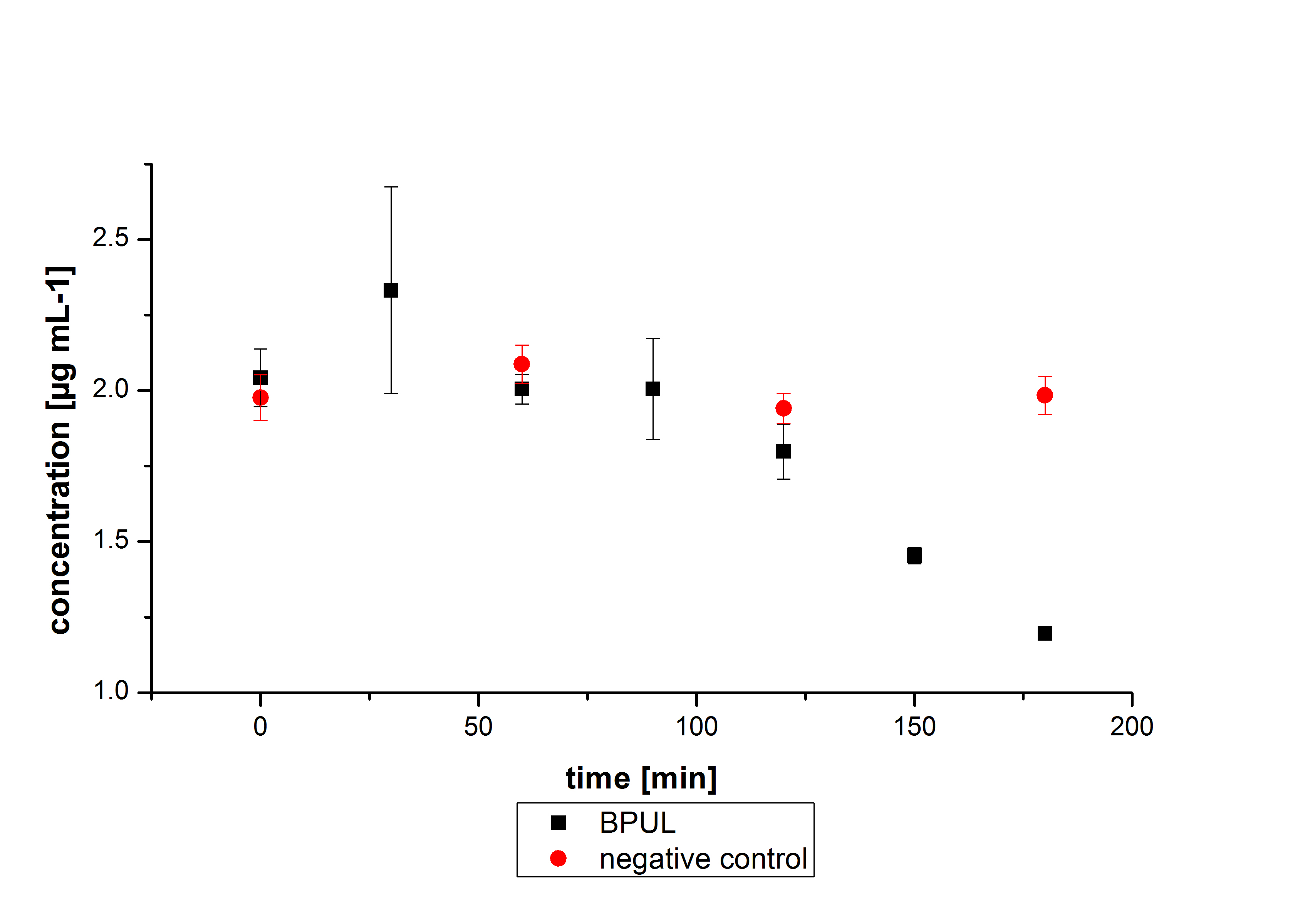
The results of our negative controls of the first Polycyclic aromatic hydrocarbon, PAH, degradations showed, that PAH´s decay without laccase with a high speed. So we take a closer look at the three PAH´s in Britton Robinson (BR)-buffer. The result can be seen in figure 2.1. After one hour most of the used substrates decayed.
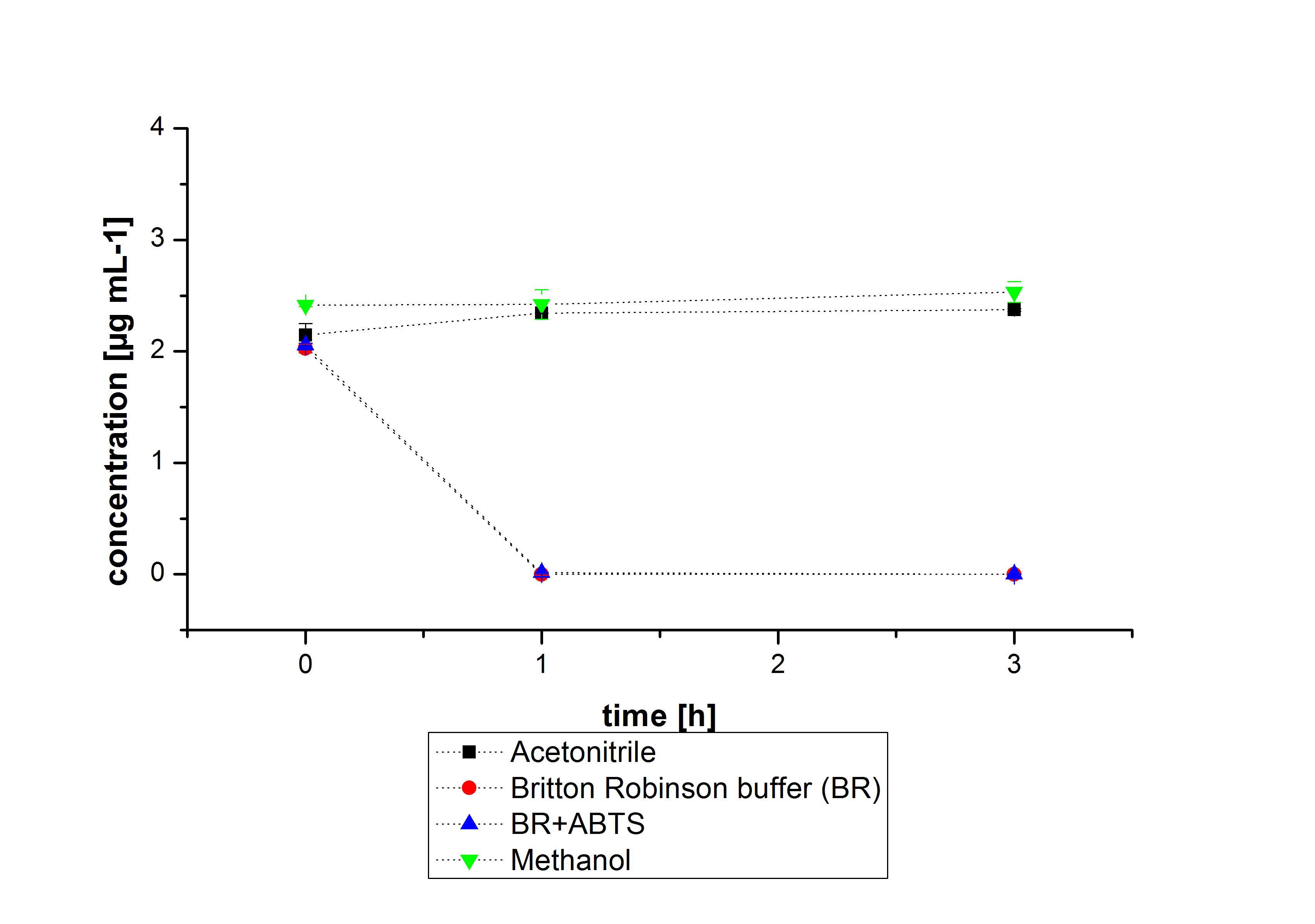
The next step was to check if and which substance cause the decay. In this test we dissolved naphthalene in acetonitrile and in methanol and compared the pure solvents with the influence of the BR-buffer and BR-buffer with ABTS. With pure methanol or acetonitrile naphthalene decays slow in comparison to BR-buffer. In BR-buffer with or without ABTS the decay happens faster and under nearly the same velocity. So BR-buffer seems to be a bad choice to test if our laccases degrade PAH´s.
Estradiol and ethinyl estradiol did not decay in BR-buffer, as shown by the negative controls in degradation experiments.
Degradation
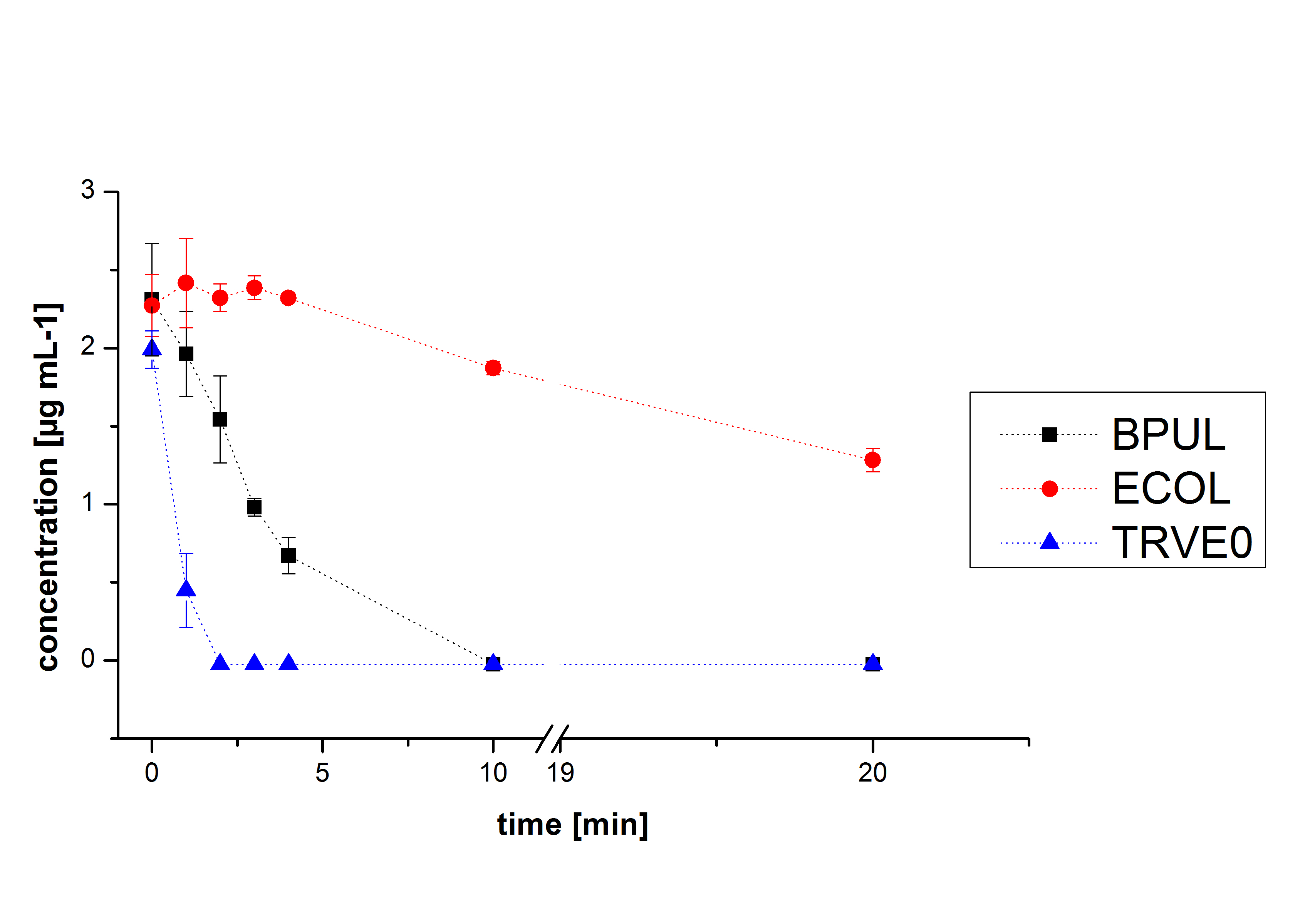
Degradation reactions with ABTS showed the expected results. "Team Activity Test" already showed, that the laccases have the ability to oxidise ABTS. The fact that oxidised ABTS reacts chemicaly with the substrates, explains that all of our active laccases have the ability to degrade ethinly estradiol and other substrates. The potential of the purchased laccase TVEL0 is much higher, shown in figure 3.2. BPUL has not the same potential as the purchased laccase. But BPUL degrades estradiol without the influence of ABTS.
Outlook
Anthracene, lindane and diclofenac could be detected with the HPLC. Ibuprofen and naproxen need an improvement of the calculated calibration curve. For naphthalene, acenaphthene and phenantrene we have to test different buffers or lower the temperature too keep it more stable. We have only tested BPUL and ECOL so far. "Team Activity Test" showed that TTHL is also active so we can test this laccase for the different substrates. Further there will be the trametis versicolor laccases TVEL5, TVEL10, TVEL13 and TVEL20. Additional we want to determine the values kcat and km for the degradable substances. Using the LC-MS we did not measure our substrates with our self producedlaccases. If there will be more time granted, we would test our self produced laccases with the substrates mentioned above.
| 55px | | | | | | | | | | |
 "
"