Team:Paris Bettencourt/Delay
From 2012.igem.org
(→Characterisation of the YiaGp promoter) |
(→Results) |
||
| Line 90: | Line 90: | ||
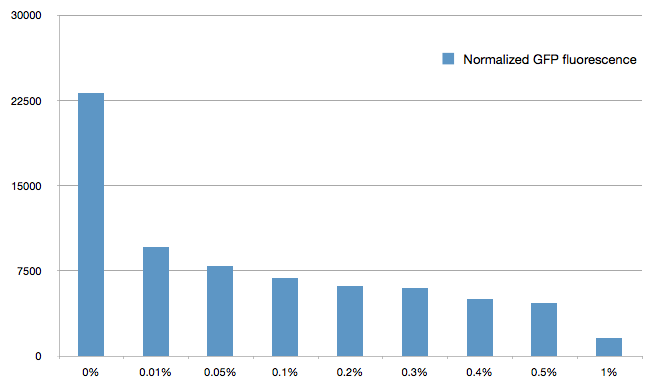
[[File:Paris_Bettencourt_2012_barplot_sRNA.png|thumb|center|600px|Normalized Fluorescence of GFP with different concentrations of arabinose]] | [[File:Paris_Bettencourt_2012_barplot_sRNA.png|thumb|center|600px|Normalized Fluorescence of GFP with different concentrations of arabinose]] | ||
| - | |||
| - | |||
| - | |||
= References = | = References = | ||
Revision as of 22:57, 26 September 2012
|
Aim : We want to provide a timespan for the cells to perform a function before triggering the DNA degradation mechanism Experimental system: We used two different approaches to create this delay. The first one is based on a simple regulatory network and eventually allows the production of the restriction enzyme I-SceI. The second one allows a immunity protein free design. The toxin is under the control of a stationary phase promoter, and repressed at a post-translational level with a sRNA induced by arabinose. Once the cells enter stationary phase, if there is no arabinose in the medium, the toxin is activated. Achievements :
|
Contents |
Overview
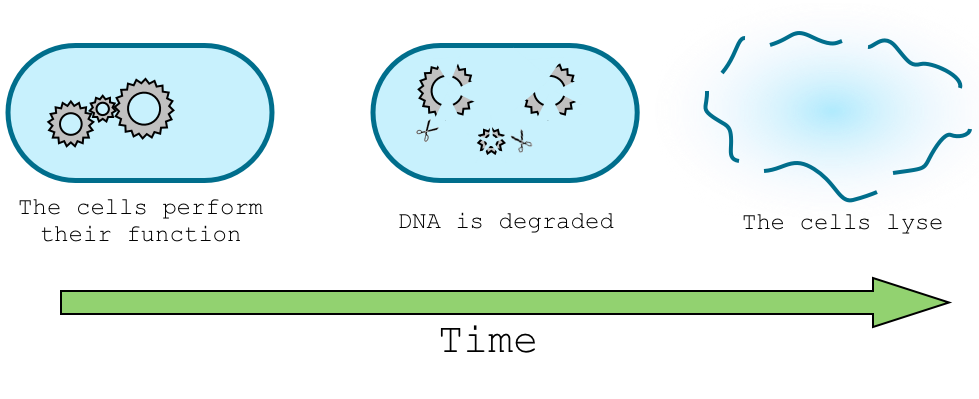
The delay system serves to suppress the function of the suicide device and preserve the integrity of the host genome while the organism does its intended job in the environment. We experimented with two designs for producing a programmed delay before the expression of the DNA-degrading suicide machinery.
The dilution delay system relies on the lab-specific expression of a transcriptional inhibitor. This inhibitor is induced by a specific compound found in the laboratory but not in the environment. When the cell enters the environment the inhibitor is gradually degraded or diluted by cell growth. Eventually, the repressor concentration falls below a critical threshold, releasing the suicide machinery.
The sRNA delay system makes use of a stationary-phase specific promoter. Under laboratory conditions, the suicide machinery is repressed by an inducible sRNA. In the environment, the suicide machinery is expressed as soon as the cells reach stationary phase.
Simple delay system
Objectives
We wanted to create a simple way to trigger the production of our restriction enzyme, leading to an inevitable death for the cells. The challenges of such a genetic circuit are the following :
- We need to have a long enough delay for the cells to perform their function before activating the "killswitch".
- We need a very strong repression (without leakage) of the restriction enzyme while the safety system is not activated.
- Our inducer should be both cheap and non toxic
Design
We chose to base our delay on a simple regulatory network : the arabinose activated promoter, pBAD, drives the production of the LacI gene, resulting in the shutdown of the expression of our restriction enzyme through a repression of the pLac promoter. As arabinose diffuses through the membrane of our alginate beads, its concentration goes down, allowing the expression of the restriction enzyme.
The system used for the characterization of this subproject is described in the figure below :
We chose pLac because it is known to be very tightly repressed by the LacI protein when this one is produced at a high level, and in the absence of lactose. This way we can ensure that the plasmid will not be prematurely degraded.
Characterization
sRNA delay system
Objectives
As an alternative trigger to the «collective suicide» presented in the overview of the project, we tried to build a system in which the toxin would be cloned without the Immunity protein, in order to avoid any possible resistance to the toxin due to a non-degraded plasmid. Such a design comes with several difficulties :
- we need a very strong repression of the expression of the toxin. A leaky expression would harm the cells, and one of the risks is that mutants which do not produce the toxin arise and become selected.
- we need to be able to have a control on the delay, in order for the cells to trigger the safety mechanism only once they have performed their function.
- we need to be sure that the safety system will be triggered at one point. We prioritize the robustness of the delay over its timespan.
Design
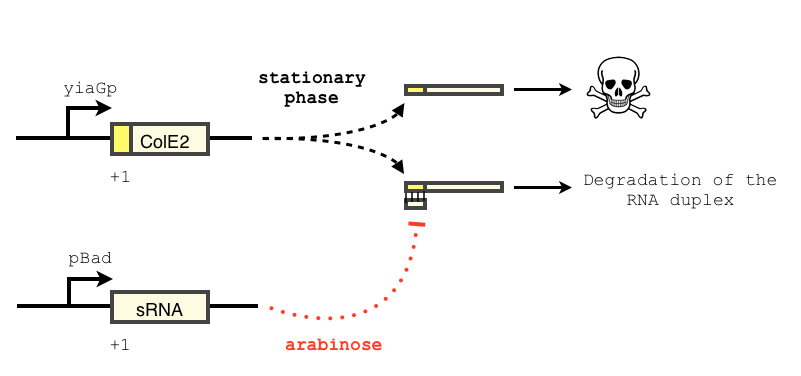
We want to use a sRNA as a way to block the expression of the toxin. The concept of our design is described below. Similar systems have been shown to respond in a treshold-linear way [2]
In order to achieve our goal, we started form the construct of Yokobayashi et al. [1] described below.
Even though the cloning is still in process, we hope to build the following construct by the end of the competition.
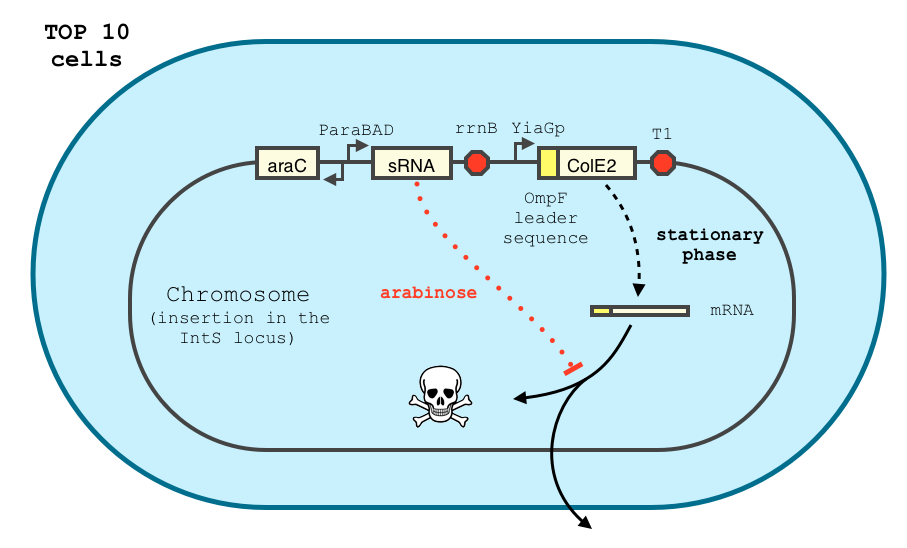
The transcription of the toxin is controlled twice : first, we chose a stationary phase promoter, yiaGp. It is known be recognized by the sigma-S subunit of the RNA polymerase, and not the exponential phase sigma-70 subunit. [3], [4].
We have shown that the yiaGp promoter is indeed activated during the stationary phase in our construct, even though the level of trancription is rather low (see experiments)
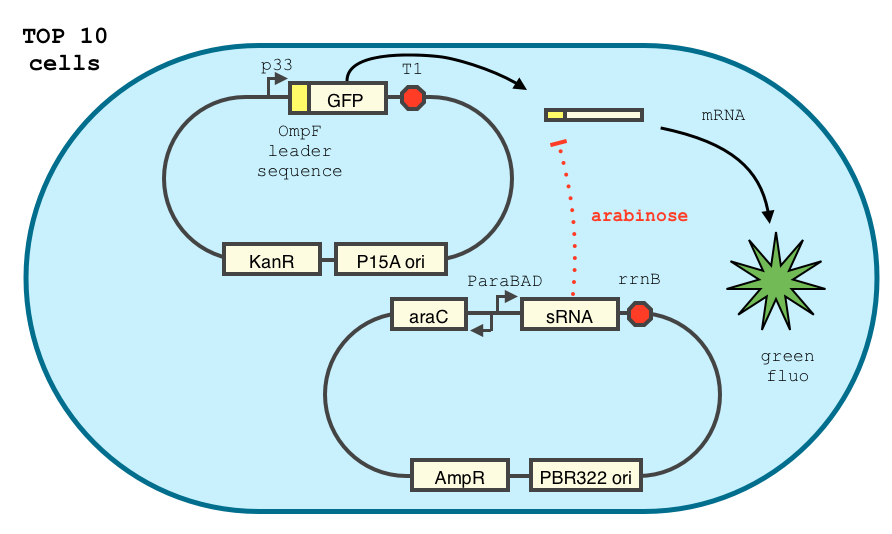
In order to achieve a complete lockdown of the toxin, we added a second repression mechanism, this time at the post transcriptional level. We used and modified the constructs of Yokobayashi et al. to allow a repression of the translation using sRNA [1]. We chose to use the sRNA 7.9, as it has been shown to bind the leader sequence of its target mRNA and not its coding sequence, and to allow a 20 fold repression of the protein’s expression. The transcription of the sRNA is under the control of the pBAD promoter. The coding sequence of colicin E2 is fused to the leader sequence (5’ UTR) of OmpF. The araC protein allows us to use this construct in cells deleted for the arabinose operon (TOP10). We hope that, using this type of cells, the delay will depend on the dilution of the arabinose in the medium as it is not metabolized. We will need to check the effect of extracellular glucose on the level of repression, as the CRP protein is known to repress the pBAD promoter.
Characterisation of the YiaGp promoter
Using the following construct, we characterized the stationary phase YiaGp promoter in TOP10 cells.
YiaGp is a late stationary phase promoter. It has been shown to be activated by 10 to 30 fold during to stationary phase, and is among the strongest promoters during this phase. Furthermore, contrary to other stationary phase promoters, its action lasts even during the late
Experimental setup
Results
Present your results
Refined characterization of the Yokobayashi et al. sRNA repression plasmidic device
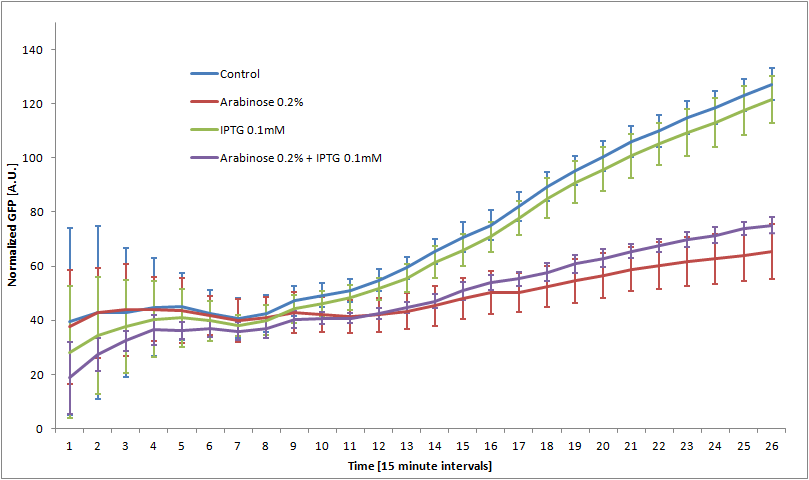
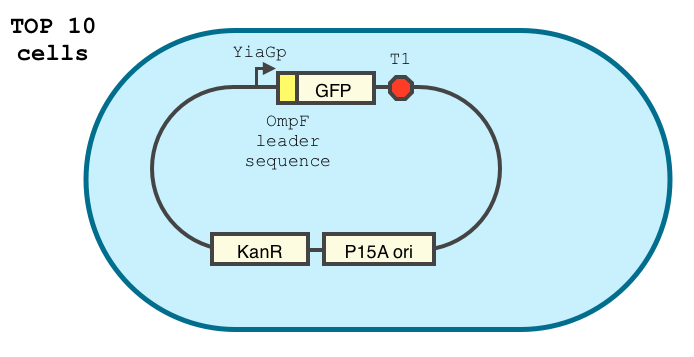
We double transformed TOP10 cells with the pKP33-GFPuv and pBAD-aOmpF-7.9 plasmids (see [1] for details, and click here for a schematic of the genetic system). We chose the 7.9 sRNA because it seemed to show a strong repression of the GFP expression, and bind to the RBS of the aOmpF leader sequence and not to the coding sequence.
The cells were grown in LB until stationary phase, then diluted 100-fold in LB. After adding various arabinose concentrations to the medium, we monitored the fluorescence of GFP (excitation : 395nm, emission : 509nm) using a TECAN 96-wells plate reader during 10 hours . We had 6 replicates for each concentration. The bar plot below shows the average normalized fluorescence at the final time point for each concentration tested.
References
1. Sharma, V., Yamamura, A., & Yokobayashi, Y. (2012). Engineering Artificial Small RNAs for Conditional Gene Silencing in Escherichia coli, 6-13. [http://pubs.acs.org/doi/abs/10.1021/sb200001q| Paper]
2. Levine, E., Zhang, Z., Kuhlman, T., & Hwa, T. (2007). Quantitative characteristics of gene regulation by small RNA. PLoS biology, 5(9), e229. doi:10.1371/journal.pbio.0050229 [http://www.plosbiology.org/article/info%3Adoi%2F10.1371%2Fjournal.pbio.0050229| Paper]
3. Sharma, U. K., & Chatterji, D. (2010). Transcriptional switching in Escherichia coli during stress and starvation by modulation of sigma activity. FEMS microbiology reviews, 34(5), 646-57. doi:10.1111/j.1574-6976.2010.00223.x[http://jb.asm.org/content/186/21/7112.long| Paper]
4. Shimada, T., Makinoshima, H., Ogawa, Y., Miki, T., Maeda, M., & Ishihama, A. (2004). Classification and Strength Measurement of Stationary-Phase Promoters by Use of a Newly Developed Promoter Cloning Vector, 186(21), 7112-7122. doi:10.1128/JB.186.21.7112 [http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6976.2010.00223.x/abstract;jsessionid=8C98B1B383242B9DFF45429802F48428.d02t04| Paper]
 "
"


 Overview
Overview Delay system
Delay system Semantic containment
Semantic containment Restriction enzyme system
Restriction enzyme system MAGE
MAGE Encapsulation
Encapsulation Synthetic import domain
Synthetic import domain Safety Questions
Safety Questions Safety Assessment
Safety Assessment