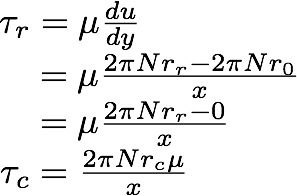Team:University College London/Module 2/Characterisation
From 2012.igem.org
(→Characterisation) |
(→Characterisation) |
||
| Line 13: | Line 13: | ||
'''Small Scale Shear Device''' | '''Small Scale Shear Device''' | ||
| - | Besides the expression of curlis, we also want to ascertain the shear resistance of the biofilm formed by the curliated cells. As such, we | + | Besides the expression of curlis, we also want to ascertain the shear resistance of the biofilm formed by the curliated cells and therefore the strength of their adhesion to the plastic itself. |
| + | |||
| + | As such, we have worked with the Engineering Faculty Rapid Design and Fabrication Facility (RDFF) to design and construct a small scale shear device which is used to quantitatively analyse the critical shear force that can be applied to biofilms expressing the UCL '12 curli construct (K729XXX) before there dettachment from the surface of plastics, and as such tell us how strong the bond between cell and plastic are due to the curli fibrils. | ||
| + | |||
| + | The device creates a variable shear gradient across the surface of the biofilm at a single rotational speed, thereby allowing us to ascertain the critical shear stresses at which our cells adhere and detach from the plastic <span class="footnote" title="Shear">surfaces</span>. | ||
The following equation allows us to determine the degree of shear stress our cells are exposed to in our experimental shear device: | The following equation allows us to determine the degree of shear stress our cells are exposed to in our experimental shear device: | ||
Revision as of 20:30, 26 September 2012
Description | Design | Construction | Characterisation | Shear Device | Modelling | Results | Conclusions
Characterisation
Congo Red Assay
The characterisation of curli expression will be done via a Congo Red agar assay. Congo Red is a diazo dye that causes cells expressing curlis to be stained red. Hence, Congo Red agar provides a convenient assay to determine if the expression of curlis has been successful.
The linear structure of the amyloid curli fibril allows the formation of hydrogen bonds with the Congo Red agar, while alternative structures are forced to form ionic bonds. These ionic bonds allow the rapid dissipation of the dye colour, resulting in the formation of pale plaques where curli negative growth occurs. The hydrogen bonds do not facilitate this removal of the dye colour, hence their growth as red colonies. This assay acts as a highly effective indicator of Curli expression.
Small Scale Shear Device
Besides the expression of curlis, we also want to ascertain the shear resistance of the biofilm formed by the curliated cells and therefore the strength of their adhesion to the plastic itself.
As such, we have worked with the Engineering Faculty Rapid Design and Fabrication Facility (RDFF) to design and construct a small scale shear device which is used to quantitatively analyse the critical shear force that can be applied to biofilms expressing the UCL '12 curli construct (K729XXX) before there dettachment from the surface of plastics, and as such tell us how strong the bond between cell and plastic are due to the curli fibrils.
The device creates a variable shear gradient across the surface of the biofilm at a single rotational speed, thereby allowing us to ascertain the critical shear stresses at which our cells adhere and detach from the plastic surfaces.
The following equation allows us to determine the degree of shear stress our cells are exposed to in our experimental shear device:
τr = Shear stress at radius r [N.m-2]
τc = Shear stress at critical radius [N.m-2]
μ = Viscosity of fluid [N.s.m-2]
N = Rotational speed of the shear device [s-1]
r = Distance from the centre of the disc [m]
x = Distance between the top and bottom disc [m]
Hence by determining the critical shear radius at which cells begin to detach, we can determine the critical shear stress.
 "
"