Team:NTU-Taida/Project/Stability
From 2012.igem.org
(Created page with "{{:Team:NTU-Taida/Templates/Header}}{{:Team:NTU-Taida/Templates/Navbar}}{{:Team:NTU-Taida/Templates/Sidebar|Title=Stability}}{{:Team:NTU-Taida/Templates/ContentStart}} {{:Team:N...") |
|||
| Line 3: | Line 3: | ||
{{:Team:NTU-Taida/Templates/BSHero|Title=Stability|Content=}} | {{:Team:NTU-Taida/Templates/BSHero|Title=Stability|Content=}} | ||
| + | ==Stability of Delivery System== | ||
| + | ===Briefing=== | ||
| + | As our PepdEx system consists of several plasmids and will function outside of the laboratory (human gut) | ||
| + | |||
| + | which lacks of antibiotic selection pressure, plasmid segregation stability is critical that determines | ||
| + | |||
| + | whether every single E. Coli cell contains the original system with the designated function. Inspired by | ||
| + | |||
| + | natural plasmid & mobile gene element, we cope with segregation instability by incorporating three | ||
| + | |||
| + | modules, i.e. '''partition system''', '''Multimer resolution system''' and '''toxin antitoxin system''', on | ||
| + | |||
| + | top of our system. We think that this layer of regulation will potentially benefit the entire synthetic | ||
| + | |||
| + | biology community, as synthetic systems are becoming increasingly complex with fine controls (e.g. on low copy | ||
| + | |||
| + | plasmids). | ||
| + | |||
| + | ===Obstacles=== | ||
| + | Sources of plasmid instability: | ||
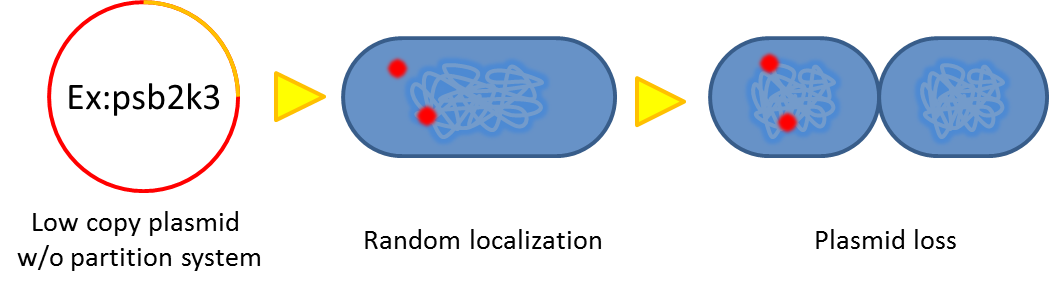
| + | # Segregational instability <p> Plasmids are randomly (possibly uneven) distributed inside a bacterium. After | ||
| + | |||
| + | cell division, one of the progenies may lose plasmids.</p>[[File:NTU-Taida-Par1.png|500px|center]] | ||
| + | # Burden Effect <p>Cells bearing our PepdEx system have to spend extra energy/resource, so will not grow as | ||
| + | |||
| + | good as plasmid-free cells. This metabolic burden will cause our coli to gradually lose pieces of circuits, | ||
| + | |||
| + | and the growth rate difference will eventually eliminate plasmid-bearing bacteria in the population.</p> | ||
| + | |||
| + | [[File:NTU-Taida-Burden3.png|500px|center]] | ||
| + | # Dimer Catastrophe<p>Homologues recombination may cause plasmid multimers which will increases segregational | ||
| + | |||
| + | instability and burden. This is the reason why most lab coli are recA1 mutants. But since our coli must be | ||
| + | |||
| + | able to colonize in the gut, it should be recA+ wild type strain. That's the problem!</p>[[File:NTU-Taida- | ||
| + | |||
| + | Mrs-1.png|500px|center]] | ||
| + | |||
| + | ===Stabilization Modules=== | ||
| + | |||
| + | ====1. Multimer Resolution System:Tn1000(gamma delta) resolution system==== | ||
| + | |||
| + | [[FIle:NTU-Taida-Mrs-2.png|400px|thumb|right|structure of MRS]] | ||
| + | |||
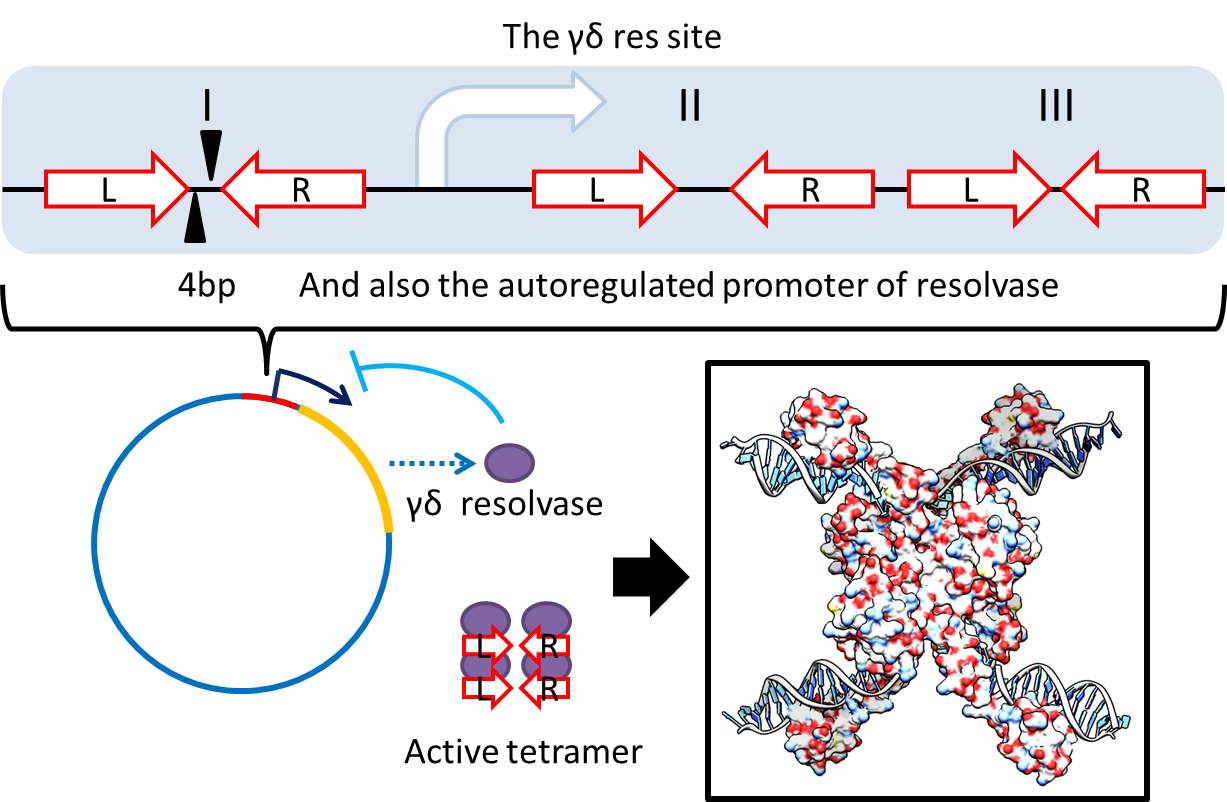
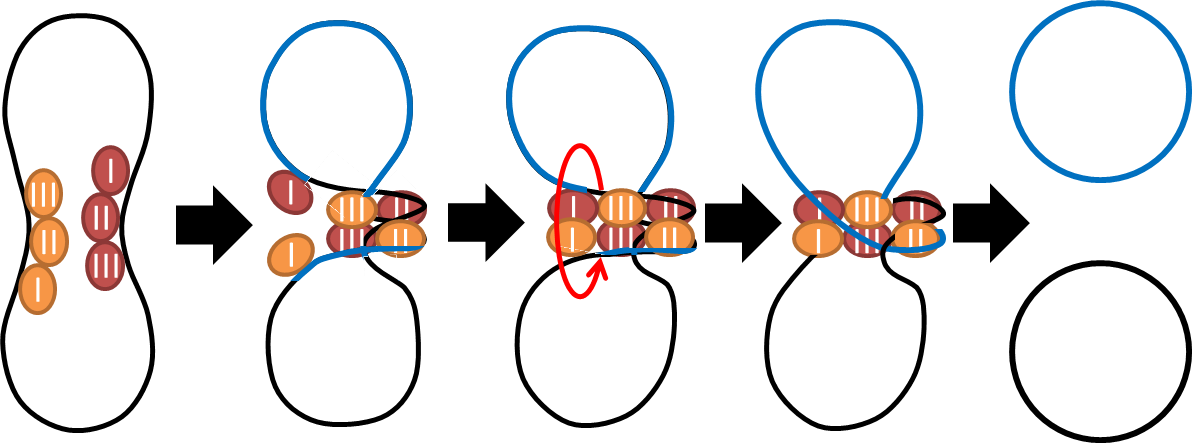
| + | :To deal with multimerization, we clone an cassette which encodes an autoregulated resolvase(serine type | ||
| + | |||
| + | recombinase)from E.coli F plasmid transposon tn1000(tn3 family). Its promoter region consists of 3 sub-sites | ||
| + | |||
| + | (res site) and can process recombination. This cassette can resolve multimer that formed during replicative | ||
| + | |||
| + | transposition, so it can also resolve plasmid multimer providing plasmid stability by avoiding dimer | ||
| + | |||
| + | catastrophe. For this reason, it is multimer resolution system(MRS) that provide analogous function as E.coli | ||
| + | |||
| + | chromosome XerCD/dif but acts independent of cell cycle, DNA localization and may have higher efficiency on | ||
| + | |||
| + | plasmids compared with slow XerCD system. | ||
| + | |||
| + | [[FIle:NTU-Taida-Mrs-4.png|200px|thumb|center]] | ||
| + | |||
| + | [[FIle:NTU-Taida-Mrs-3.png|600px|thumb|center]] | ||
| + | |||
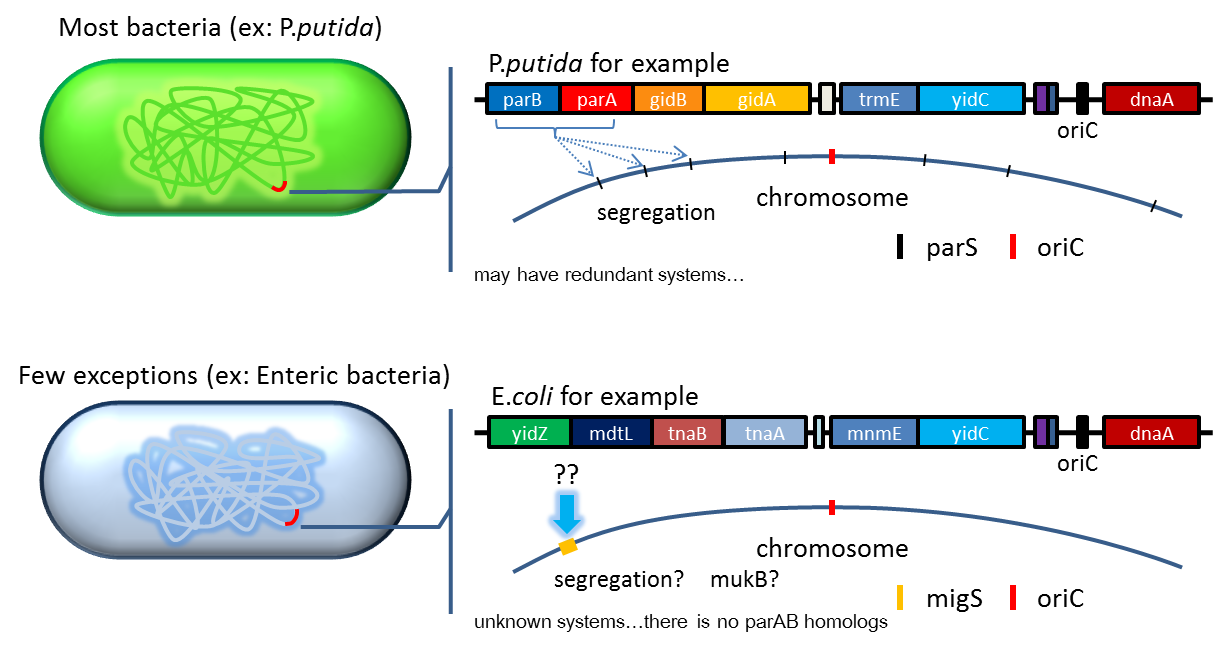
| + | ====2. Partition System:from '''Pseudomonas putida'''KT2440==== | ||
| + | |||
| + | :Just like many low copy plasmid, bacterial chromosome distribute chromosome evenly to their progeny by | ||
| + | |||
| + | certain dynamic system. These systems include SMC like proteins, type Ia partition system and so on. Type Ia | ||
| + | |||
| + | partition system segregate chromosome/plasmid in a process akin to Eukaryotic mitosis,it can be found on most | ||
| + | |||
| + | eubacteria but E.coli is not the case. | ||
| + | |||
| + | [[FIle:NTU-Taida-Par2.png|700px|thumb|center]] | ||
| + | |||
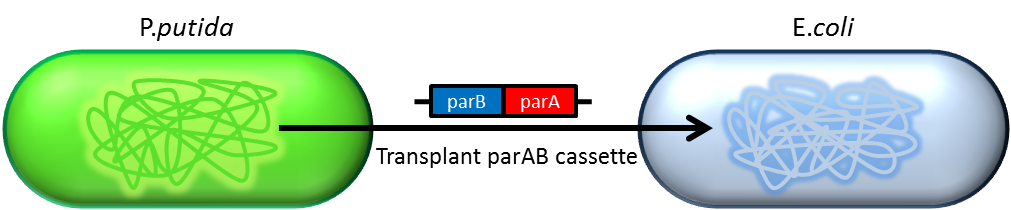
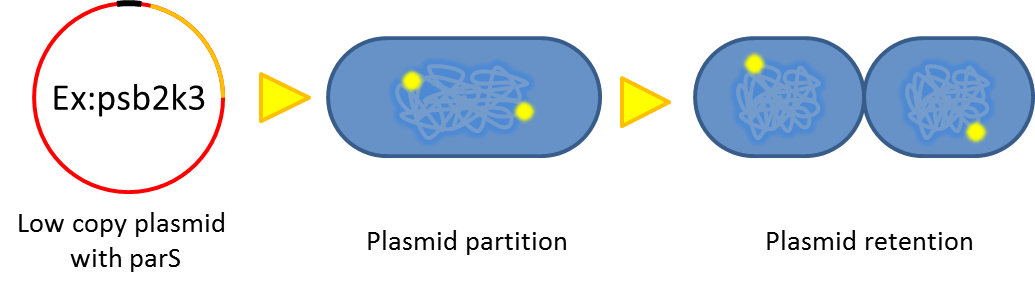
| + | :Previous study have shown that when provide parAB of P.putida in trans, the low copy plasmid(mini F)carrying | ||
| + | |||
| + | conserved parS site can be stabilized in E.coli.(Anne-Marie etl. 2002)pSB2K3 is mini F plasmid thus can be | ||
| + | |||
| + | stabilized by this way too, we make a new version of pSB2K3 with parS site. This plasmid that have lower gene | ||
| + | |||
| + | dosage(copy number)and can be partitioned is an ideal vector to harbor our pepdEX system. | ||
| + | |||
| + | [[FIle:NTU-Taida-Par3.png|500px|thumb|center]] | ||
| + | |||
| + | [[FIle:NTU-Taida-Par4.png|500px|thumb|center]] | ||
| + | |||
| + | ====3. Post-Segregation Killing:srnBC toxin-antitoxin system==== | ||
| + | |||
| + | :No matter how well partition system and multimer resolution system work, inevitably there still will have | ||
| + | |||
| + | some bacteria losing plasmid. We sentence them to death to solve the problem. | ||
| + | |||
| + | {| align="center" | ||
| + | | [[FIle:NTU-Taida-TAgrenade.png|200px]] | ||
| + | | [[FIle:NTU-Taida-bacteria-work-hard.png|200px|Work hard or Die hard!!!]] | ||
| + | |} | ||
| + | |||
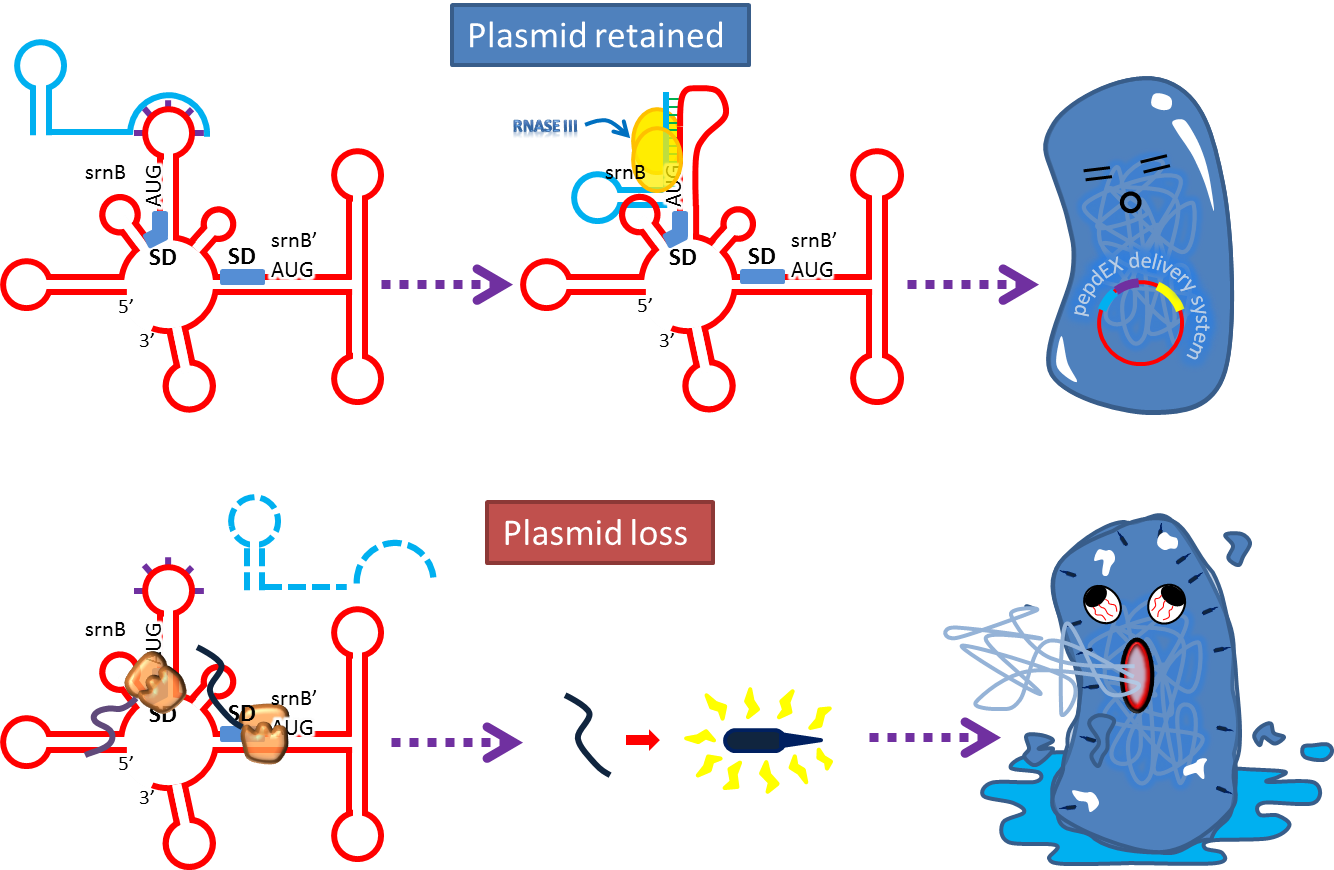
| + | :Type I toxin-antitoxin srnBC is an ideal executor which belongs to hok/sok homologues, it expresses stable | ||
| + | |||
| + | toxin encoded RNA and short lived antitoxin RNA that can neutralize toxin RNA by RNA interaction and RNase III | ||
| + | |||
| + | cleavage. It acts as post segregation killing system, which kills bacteria when it loss the DNA(genomic | ||
| + | |||
| + | islands, plasmids, mobile gene elements) that contains it. Therefore we use it to reduce plasmid loss rate and | ||
| + | |||
| + | make applications without antibiotic selection more feasible. | ||
| + | |||
| + | [[FIle:NTU-Taida-SrnBC-Mechanism1.png|600px|thumb|center|TA loci expresses two kinds of RNA which is srnB and | ||
| + | |||
| + | srnC respectively. Initially, srnB is inactive and stable (half-life in the order of 30 min[9]) and will | ||
| + | |||
| + | gradually be processed to active form producing hydrophobic toxic protein if srnC RNA does not exist; srnC is | ||
| + | |||
| + | relative unstable and degrade quickly, it overlaps and compliment to 5’sequence of srnB.]] | ||
| + | |||
| + | [[FIle:NTU-Taida-SrnBC-Mechanism2.png|600px|thumb|center|At normal condition (plasmid retained), srnC paired | ||
| + | |||
| + | with processed srnB and forming double stranded RNA that is susceptible to RNase III cleavage and thus prevent | ||
| + | |||
| + | toxin production. If plasmid loss, srnC will quickly disappeared without replenishing and srnB will show its | ||
| + | |||
| + | power[10].]] | ||
| + | |||
| + | ====4. Reduce Burden Effect==== | ||
| + | |||
| + | :Besides these modules, reducing the burden effect is also important to the system stability. Well designed | ||
| + | |||
| + | system and lower gene dosage may help. | ||
| + | : | ||
| + | :High level gene expression and gene dosage cause burden effect. If having the same outcome, low copy plasmid | ||
| + | |||
| + | is preferred to high copy ones. If having the same outcome and not for regulatory purpose, stabilizing mRNA is | ||
| + | |||
| + | preferred to overexpression it. Place yourselves in E.coli's position, reduce its metabolic burden as much as | ||
| + | |||
| + | possible then it can work for you. | ||
| + | |||
| + | [[FIle:NTU-Taida-Burden2.png|350px|center]] | ||
| + | |||
| + | |||
| + | === Modeling and Application === | ||
| + | ====How to model plasmid instability:==== | ||
| + | We use Cooper's model (Cooper, N.S., M.E. Brown, and C.A. Caulcott, A ) to model plasmid instability, and set | ||
| + | |||
| + | a protocol to suggest users which modules can be used to prove their system stability. Click upper button | ||
| + | |||
| + | "modeling" for detail or press following link. | ||
| + | |||
| + | [[FIle:NTU-Taida-Negative du.jpg|400px|center]] | ||
| + | |||
| + | == Safety of Delivery System == | ||
| + | Many turn off strategies have been developed, most of these are the inducible suicide system that can be | ||
| + | |||
| + | activated at certain condition. For instance, in our project, we plan to use temperature and small molecule as | ||
| + | |||
| + | activating signals( following picture). When the course of treatment ends, administration of small amount of | ||
| + | |||
| + | tetracycline agonist will induce bacterial to commit suicide, leaving human body also cause suicide gene | ||
| + | |||
| + | activation thereby avoid recombinant strain/gene polluting. And splitting suicide system to provide repression | ||
| + | |||
| + | in trans can prevent plasmid transfering to wild type strains. There have been many off-the-rack parts can be | ||
| + | |||
| + | used. | ||
| + | |||
| + | [[File:NTU-Taida-Suicide-system.png|300px|thumb|left|alt=new idea about safety|general scheme of suicide | ||
| + | |||
| + | system ]] | ||
| + | |||
| + | However, these design cannot totally eliminate the risk of horizontal gene transfer(HGT), which recombinant | ||
| + | |||
| + | genes can move to other organisms independent of suicide system. So besides suicide system, we have a new idea | ||
| + | |||
| + | to deal with these kinds of HGT risks by RNA interaction. | ||
| + | |||
| + | Although bacteria lack for RNAi pathway, expressing well designed antisense RNAs have been shown to have | ||
| + | |||
| + | inhibitory effect on target RNAs through competitive inhibition, and recent study showed that peptide nucleic | ||
| + | |||
| + | acid (PNA) that antisense to antitoxin RNA 5' sequence can cause bacteria death. Putting appropriate antisense | ||
| + | |||
| + | RNAs on untranslated region of transcripts may interfere target RNA function or translation. This property | ||
| + | |||
| + | might be used to prevent HGT. For instance, HGT is more likely to occur between related species like lab | ||
| + | |||
| + | E.coli & O157, laboratory E.coli have inactivated all its hok/sok toxin-antitoxin system by mutation, but | ||
| + | |||
| + | wild bacteria especially pathogenic bacteria usually have more active TA locus on its chromosome like E.coli | ||
| + | |||
| + | O157. we plan to put a stem loop from hok mRNA which can pair with sok RNA 5’sequence on UTR of antibiotic | ||
| + | |||
| + | resistance genes we used in pepdEX system. IF wild bacteria steal our antibiotic resistance genes and express | ||
| + | |||
| + | it, its antitoxin will be competitive inhibited and its toxin will express and kill the thief thus preventing | ||
| + | |||
| + | HGT between lab & wild coli. This idea can have wide extension. Besides targeting antitoxin (functional | ||
| + | |||
| + | RNA) of type I TA, designing antisense sequences that target RBS to down regulate targeted protein is also | ||
| + | |||
| + | possible. Targeting antitoxin of type II TA, essential genes for metabolism, housekeeping genes and any | ||
| + | |||
| + | sequences exist in potential HGT receivers but not our coli can be used. Even if the design cannot kill | ||
| + | |||
| + | thieves, it can weaken receivers and reduce advantages antibiotic genes bring about thus reduce possibility | ||
| + | |||
| + | and danger of HGT. | ||
| + | |||
| + | In the past the repression efficiencies of antisense RNA in bacteria are low, but after invention of the | ||
| + | |||
| + | paired termini antisense RNA(PTasRNA) method and incorporate U turn/YUNR motif etc., this idea will become | ||
| + | |||
| + | more and more feasible. | ||
| + | |||
| + | [[File:NTU-Taida-AsRNA.png|700px|center|thumb|alt=new idea about safety|Is it possible to use RNA interaction | ||
| + | |||
| + | to avoid HGT? ]] | ||
| + | |||
| + | === Reference of Safety of Delivery System === | ||
| + | <ol> | ||
| + | <li> | ||
| + | Field, C.M. and D.K. Summers, Multicopy plasmid stability: revisiting the dimer catastrophe. Journal of | ||
| + | |||
| + | Theoretical Biology, 2011. 291: p. 119-27. | ||
| + | </li> | ||
| + | <li> | ||
| + | Grindley, N.D.F., et al., Transposon-Mediated Site-Specific Recombination - Identification of 3 Binding-Sites | ||
| + | |||
| + | for Resolvase at the Res-Sites of Gamma-Delta and Tn3. Cell, 1982. 30(1): p. 19-27. | ||
| + | </li> | ||
| + | <li> | ||
| + | Wells, R.G. and N.D.F. Grindley, Analysis of the Gamma-Delta Res Site - Sites Required for Site-Specific | ||
| + | |||
| + | Recombination and Gene-Expression. Journal of Molecular Biology, 1984. 179(4): p. 667-687. | ||
| + | </li> | ||
| + | <li> | ||
| + | Mullins, R.D., Bacterial Chromosome Segregation. Annu Rev Cell Dev Biol, 2009. | ||
| + | </li> | ||
| + | <li> | ||
| + | Godfrin-Estevenon, A.M., F. Pasta, and D. Lane, The parAB gene products of Pseudomonas putida exhibit | ||
| + | |||
| + | partition activity in both P. putida and Escherichia coli. Molecular Microbiology, 2002. 43(1): p. 39-49. | ||
| + | </li> | ||
| + | <li> | ||
| + | Gerdes, K., et al., Mechanism of killer gene activation. Antisense RNA-dependent RNase III cleavage ensures | ||
| + | |||
| + | rapid turn-over of the stable hok, srnB and pndA effector messenger RNAs. Journal of Molecular Biology, 1992. | ||
| + | |||
| + | 226(3): p. 637-49. | ||
| + | </li> | ||
| + | <li> | ||
| + | Thisted, T. and K. Gerdes, Mechanism of post-segregational killing by the hok/sok system of plasmid R1. Sok | ||
| + | |||
| + | antisense RNA regulates hok gene expression indirectly through the overlapping mok gene. Journal of Molecular | ||
| + | |||
| + | Biology, 1992. 223(1): p. 41-54. | ||
| + | </li> | ||
| + | <li> | ||
| + | Szekeres, S., et al., Chromosomal toxin-antitoxin loci can diminish large-scale genome reductions in the | ||
| + | |||
| + | absence of selection. Molecular Microbiology, 2007. 63(6): p. 1588-1605. | ||
| + | </li> | ||
| + | <li> | ||
| + | Faridani, O.R., et al., Competitive inhibition of natural antisense Sok-RNA interactions activates Hok- | ||
| + | |||
| + | mediated cell killing in Escherichia coli. Nucleic Acids Research, 2006. 34(20): p. 5915-5922. | ||
| + | </li> | ||
| + | <li> | ||
| + | Gerdes, K. and E.G.H. Wagner, RNA antitoxins. Current Opinion in Microbiology, 2007. 10(2): p. 117-124. | ||
| + | </li> | ||
| + | <li> | ||
| + | Good, L. and J.E. Stach, Synthetic RNA silencing in bacteria - antimicrobial discovery and resistance | ||
| + | |||
| + | breaking. Front Microbiol, 2011. 2: p. 185. | ||
| + | </li> | ||
| + | <li> | ||
| + | Faridani, O.R., et al., Competitive inhibition of natural antisense Sok-RNA interactions activates Hok- | ||
| + | |||
| + | mediated cell killing in Escherichia coli. Nucleic Acids Research, 2006. 34(20): p. 5915-22. | ||
| + | </li> | ||
| + | <li> | ||
| + | Lucks, J.B., et al., Versatile RNA-sensing transcriptional regulators for engineering genetic networks. Proc | ||
| + | |||
| + | Natl Acad Sci U S A, 2011. 108(21): p. 8617-22. | ||
| + | </li> | ||
| + | <li> | ||
| + | Franch, T., et al., Antisense RNA regulation in prokaryotes: rapid RNA/RNA interaction facilitated by a | ||
| + | |||
| + | general U-turn loop structure. Journal of Molecular Biology, 1999. 294(5): p. 1115-25. | ||
| + | </li> | ||
| + | </ol> | ||
<!-- EOF --> | <!-- EOF --> | ||
{{:Team:NTU-Taida/Templates/ContentEnd}}{{:Team:NTU-Taida/Templates/Footer|ActiveNavbar=Project, #nav-Project-Stability}} | {{:Team:NTU-Taida/Templates/ContentEnd}}{{:Team:NTU-Taida/Templates/Footer|ActiveNavbar=Project, #nav-Project-Stability}} | ||
Revision as of 17:31, 26 September 2012
Stability
Contents |
Stability of Delivery System
Briefing
As our PepdEx system consists of several plasmids and will function outside of the laboratory (human gut)
which lacks of antibiotic selection pressure, plasmid segregation stability is critical that determines
whether every single E. Coli cell contains the original system with the designated function. Inspired by
natural plasmid & mobile gene element, we cope with segregation instability by incorporating three
modules, i.e. partition system, Multimer resolution system and toxin antitoxin system, on
top of our system. We think that this layer of regulation will potentially benefit the entire synthetic
biology community, as synthetic systems are becoming increasingly complex with fine controls (e.g. on low copy
plasmids).
Obstacles
Sources of plasmid instability:
- Segregational instability
Plasmids are randomly (possibly uneven) distributed inside a bacterium. After
- Burden Effect
Cells bearing our PepdEx system have to spend extra energy/resource, so will not grow as
good as plasmid-free cells. This metabolic burden will cause our coli to gradually lose pieces of circuits,
and the growth rate difference will eventually eliminate plasmid-bearing bacteria in the population.- Dimer Catastrophe
Homologues recombination may cause plasmid multimers which will increases segregational
instability and burden. This is the reason why most lab coli are recA1 mutants. But since our coli must be
able to colonize in the gut, it should be recA+ wild type strain. That's the problem![[File:NTU-Taida-Mrs-1.png|500px|center]]
Stabilization Modules
1. Multimer Resolution System:Tn1000(gamma delta) resolution system
- To deal with multimerization, we clone an cassette which encodes an autoregulated resolvase(serine type
recombinase)from E.coli F plasmid transposon tn1000(tn3 family). Its promoter region consists of 3 sub-sites
(res site) and can process recombination. This cassette can resolve multimer that formed during replicative
transposition, so it can also resolve plasmid multimer providing plasmid stability by avoiding dimer
catastrophe. For this reason, it is multimer resolution system(MRS) that provide analogous function as E.coli
chromosome XerCD/dif but acts independent of cell cycle, DNA localization and may have higher efficiency on
plasmids compared with slow XerCD system.
2. Partition System:from Pseudomonas putidaKT2440
- Just like many low copy plasmid, bacterial chromosome distribute chromosome evenly to their progeny by
certain dynamic system. These systems include SMC like proteins, type Ia partition system and so on. Type Ia
partition system segregate chromosome/plasmid in a process akin to Eukaryotic mitosis,it can be found on most
eubacteria but E.coli is not the case.
- Previous study have shown that when provide parAB of P.putida in trans, the low copy plasmid(mini F)carrying
conserved parS site can be stabilized in E.coli.(Anne-Marie etl. 2002)pSB2K3 is mini F plasmid thus can be
stabilized by this way too, we make a new version of pSB2K3 with parS site. This plasmid that have lower gene
dosage(copy number)and can be partitioned is an ideal vector to harbor our pepdEX system.
3. Post-Segregation Killing:srnBC toxin-antitoxin system
- No matter how well partition system and multimer resolution system work, inevitably there still will have
some bacteria losing plasmid. We sentence them to death to solve the problem.
| 
|
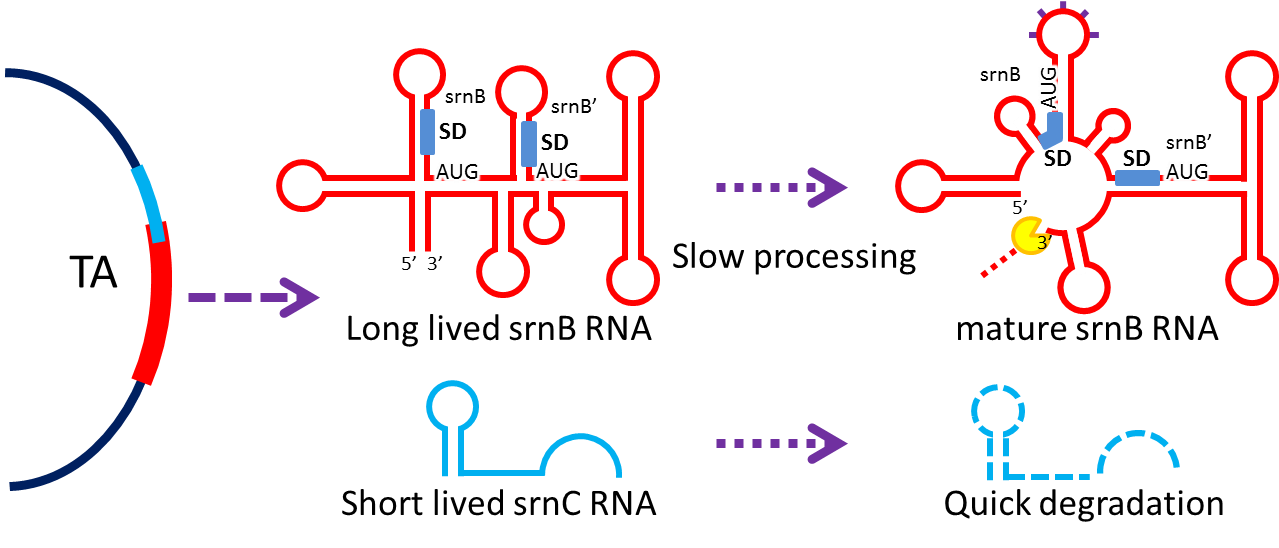
- Type I toxin-antitoxin srnBC is an ideal executor which belongs to hok/sok homologues, it expresses stable
toxin encoded RNA and short lived antitoxin RNA that can neutralize toxin RNA by RNA interaction and RNase III
cleavage. It acts as post segregation killing system, which kills bacteria when it loss the DNA(genomic
islands, plasmids, mobile gene elements) that contains it. Therefore we use it to reduce plasmid loss rate and
make applications without antibiotic selection more feasible.
4. Reduce Burden Effect
- Besides these modules, reducing the burden effect is also important to the system stability. Well designed
system and lower gene dosage may help.
- High level gene expression and gene dosage cause burden effect. If having the same outcome, low copy plasmid
is preferred to high copy ones. If having the same outcome and not for regulatory purpose, stabilizing mRNA is
preferred to overexpression it. Place yourselves in E.coli's position, reduce its metabolic burden as much as
possible then it can work for you.
Modeling and Application
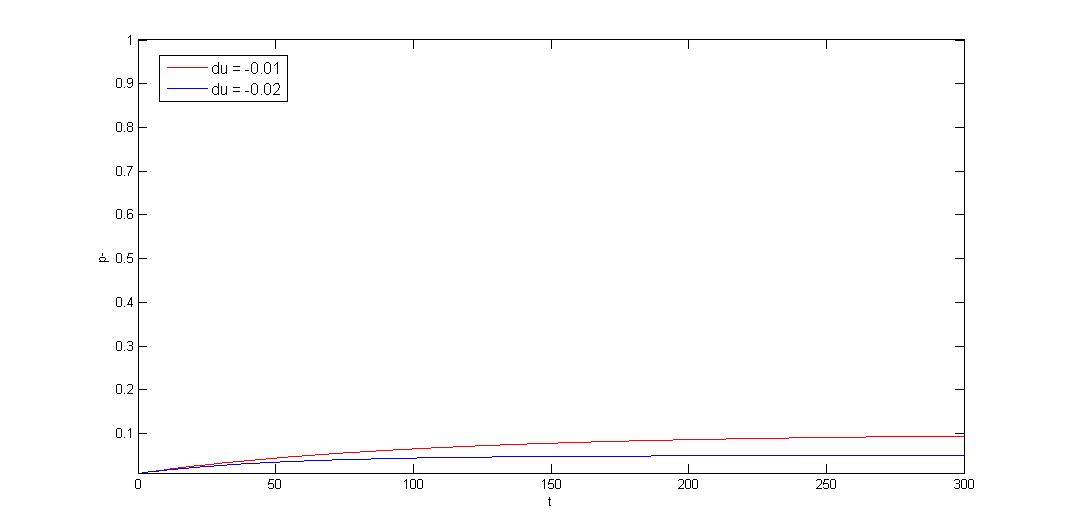
How to model plasmid instability:
We use Cooper's model (Cooper, N.S., M.E. Brown, and C.A. Caulcott, A ) to model plasmid instability, and set
a protocol to suggest users which modules can be used to prove their system stability. Click upper button
"modeling" for detail or press following link.
Safety of Delivery System
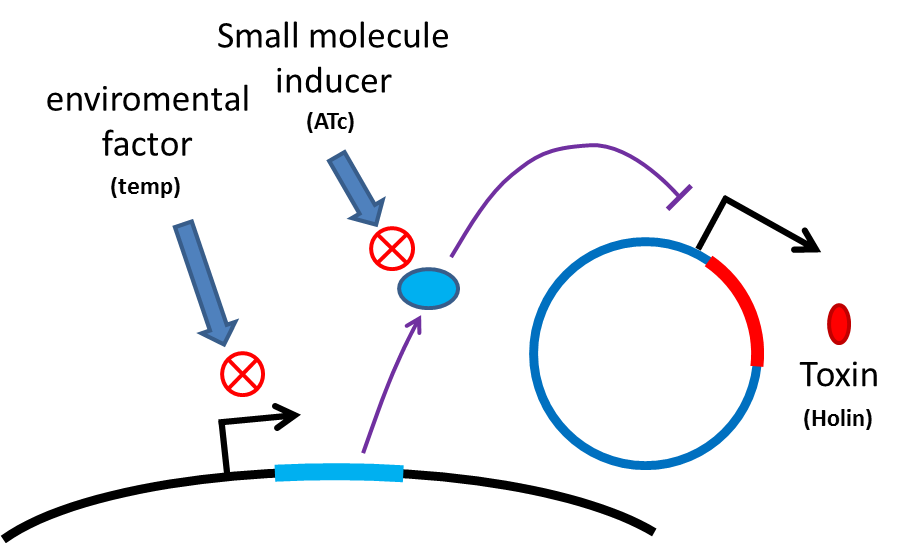
Many turn off strategies have been developed, most of these are the inducible suicide system that can be
activated at certain condition. For instance, in our project, we plan to use temperature and small molecule as
activating signals( following picture). When the course of treatment ends, administration of small amount of
tetracycline agonist will induce bacterial to commit suicide, leaving human body also cause suicide gene
activation thereby avoid recombinant strain/gene polluting. And splitting suicide system to provide repression
in trans can prevent plasmid transfering to wild type strains. There have been many off-the-rack parts can be
used.
However, these design cannot totally eliminate the risk of horizontal gene transfer(HGT), which recombinant
genes can move to other organisms independent of suicide system. So besides suicide system, we have a new idea
to deal with these kinds of HGT risks by RNA interaction.
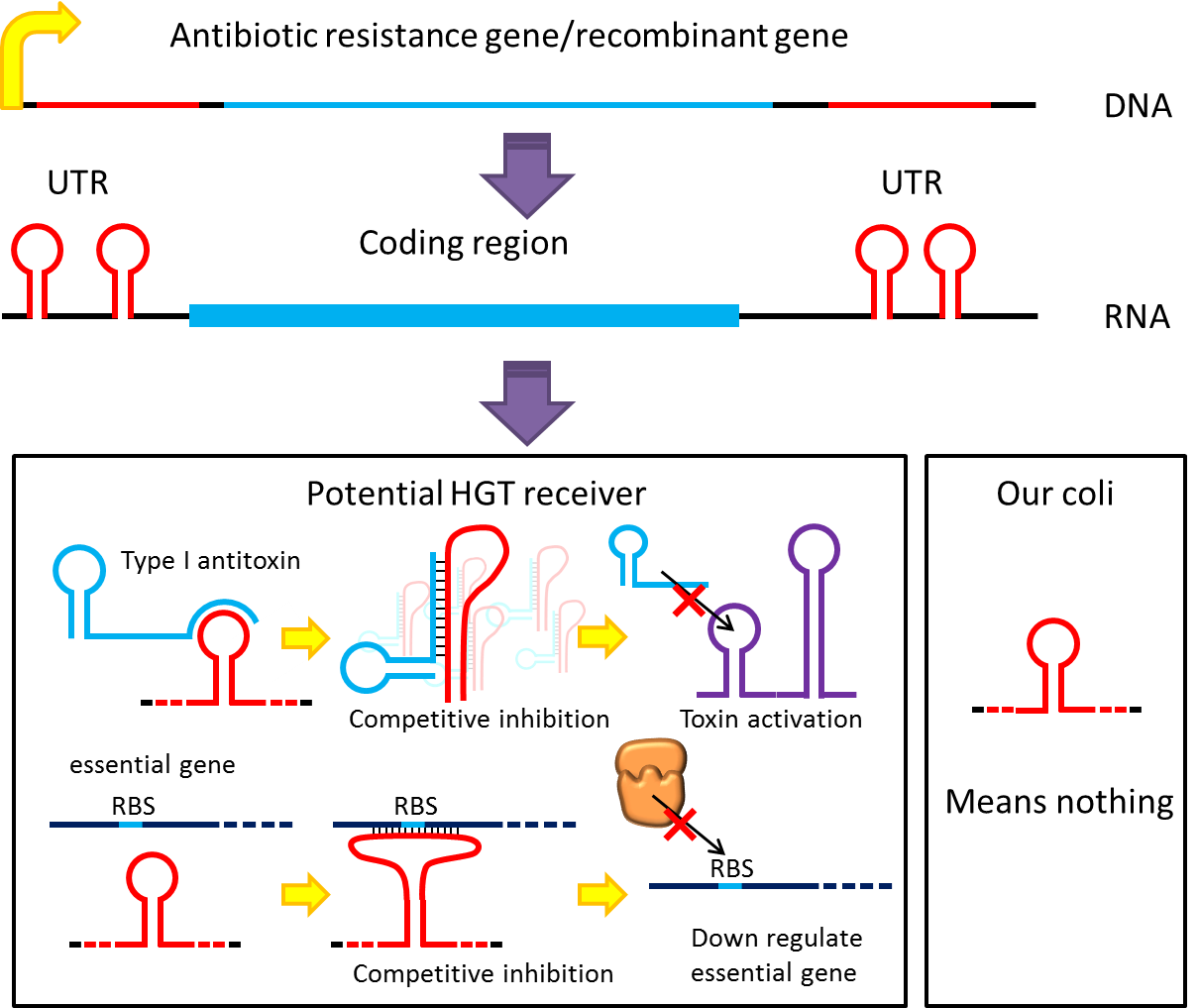
Although bacteria lack for RNAi pathway, expressing well designed antisense RNAs have been shown to have
inhibitory effect on target RNAs through competitive inhibition, and recent study showed that peptide nucleic
acid (PNA) that antisense to antitoxin RNA 5' sequence can cause bacteria death. Putting appropriate antisense
RNAs on untranslated region of transcripts may interfere target RNA function or translation. This property
might be used to prevent HGT. For instance, HGT is more likely to occur between related species like lab
E.coli & O157, laboratory E.coli have inactivated all its hok/sok toxin-antitoxin system by mutation, but
wild bacteria especially pathogenic bacteria usually have more active TA locus on its chromosome like E.coli
O157. we plan to put a stem loop from hok mRNA which can pair with sok RNA 5’sequence on UTR of antibiotic
resistance genes we used in pepdEX system. IF wild bacteria steal our antibiotic resistance genes and express
it, its antitoxin will be competitive inhibited and its toxin will express and kill the thief thus preventing
HGT between lab & wild coli. This idea can have wide extension. Besides targeting antitoxin (functional
RNA) of type I TA, designing antisense sequences that target RBS to down regulate targeted protein is also
possible. Targeting antitoxin of type II TA, essential genes for metabolism, housekeeping genes and any
sequences exist in potential HGT receivers but not our coli can be used. Even if the design cannot kill
thieves, it can weaken receivers and reduce advantages antibiotic genes bring about thus reduce possibility
and danger of HGT.
In the past the repression efficiencies of antisense RNA in bacteria are low, but after invention of the
paired termini antisense RNA(PTasRNA) method and incorporate U turn/YUNR motif etc., this idea will become
more and more feasible.
Reference of Safety of Delivery System
- Field, C.M. and D.K. Summers, Multicopy plasmid stability: revisiting the dimer catastrophe. Journal of Theoretical Biology, 2011. 291: p. 119-27.
- Grindley, N.D.F., et al., Transposon-Mediated Site-Specific Recombination - Identification of 3 Binding-Sites for Resolvase at the Res-Sites of Gamma-Delta and Tn3. Cell, 1982. 30(1): p. 19-27.
- Wells, R.G. and N.D.F. Grindley, Analysis of the Gamma-Delta Res Site - Sites Required for Site-Specific Recombination and Gene-Expression. Journal of Molecular Biology, 1984. 179(4): p. 667-687.
- Mullins, R.D., Bacterial Chromosome Segregation. Annu Rev Cell Dev Biol, 2009.
- Godfrin-Estevenon, A.M., F. Pasta, and D. Lane, The parAB gene products of Pseudomonas putida exhibit partition activity in both P. putida and Escherichia coli. Molecular Microbiology, 2002. 43(1): p. 39-49.
- Gerdes, K., et al., Mechanism of killer gene activation. Antisense RNA-dependent RNase III cleavage ensures rapid turn-over of the stable hok, srnB and pndA effector messenger RNAs. Journal of Molecular Biology, 1992. 226(3): p. 637-49.
- Thisted, T. and K. Gerdes, Mechanism of post-segregational killing by the hok/sok system of plasmid R1. Sok antisense RNA regulates hok gene expression indirectly through the overlapping mok gene. Journal of Molecular Biology, 1992. 223(1): p. 41-54.
- Szekeres, S., et al., Chromosomal toxin-antitoxin loci can diminish large-scale genome reductions in the absence of selection. Molecular Microbiology, 2007. 63(6): p. 1588-1605.
- Faridani, O.R., et al., Competitive inhibition of natural antisense Sok-RNA interactions activates Hok- mediated cell killing in Escherichia coli. Nucleic Acids Research, 2006. 34(20): p. 5915-5922.
- Gerdes, K. and E.G.H. Wagner, RNA antitoxins. Current Opinion in Microbiology, 2007. 10(2): p. 117-124.
- Good, L. and J.E. Stach, Synthetic RNA silencing in bacteria - antimicrobial discovery and resistance breaking. Front Microbiol, 2011. 2: p. 185.
- Faridani, O.R., et al., Competitive inhibition of natural antisense Sok-RNA interactions activates Hok- mediated cell killing in Escherichia coli. Nucleic Acids Research, 2006. 34(20): p. 5915-22.
- Lucks, J.B., et al., Versatile RNA-sensing transcriptional regulators for engineering genetic networks. Proc Natl Acad Sci U S A, 2011. 108(21): p. 8617-22.
- Franch, T., et al., Antisense RNA regulation in prokaryotes: rapid RNA/RNA interaction facilitated by a general U-turn loop structure. Journal of Molecular Biology, 1999. 294(5): p. 1115-25.
 "
"