Team:HokkaidoU Japan/Notebook/aggregation Week 11
From 2012.igem.org
| Line 138: | Line 138: | ||
<p> | <p> | ||
#Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. | #Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. | ||
| - | #Centrifuged | + | #Centrifuged at 14000 rpm, 30 min at 4C. |
#Remove supernatant and added 220 ul of 70% ethanol. | #Remove supernatant and added 220 ul of 70% ethanol. | ||
| - | #Centrifuged | + | #Centrifuged at 15000 rpm, 15 min at 4C. |
| - | #Remove supernatant and air drying | + | #Remove supernatant and air drying at room temperature then added 5 ul of DW. |
| Line 292: | Line 292: | ||
==Aggregation check of pBAD-RBS-eCFP-RBS-Ag43-dT-pSTV28== | ==Aggregation check of pBAD-RBS-eCFP-RBS-Ag43-dT-pSTV28== | ||
<p> | <p> | ||
| - | Aggregation check for No.5,6 colonies selected by colony PCR | + | Aggregation check for No.5,6 colonies selected by colony PCR at 11th. |
#Prepared 5 ml LBA into glass tubes. | #Prepared 5 ml LBA into glass tubes. | ||
#Re-suspended 2 colony mixture (No.2 and No.5 respectively). | #Re-suspended 2 colony mixture (No.2 and No.5 respectively). | ||
| Line 379: | Line 379: | ||
<p> | <p> | ||
#Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. | #Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. | ||
| - | #Centrifuged | + | #Centrifuged at 15000 rpm, 15 min at 4C. |
#Remove supernatant and added 220 ul of 70% ethanol. | #Remove supernatant and added 220 ul of 70% ethanol. | ||
| - | #Centrifuged | + | #Centrifuged at 15000 rpm, 10 min at 4C. |
| - | #Remove supernatant and air drying | + | #Remove supernatant and air drying at room temperature then added 10 ul of DW. |
| Line 624: | Line 624: | ||
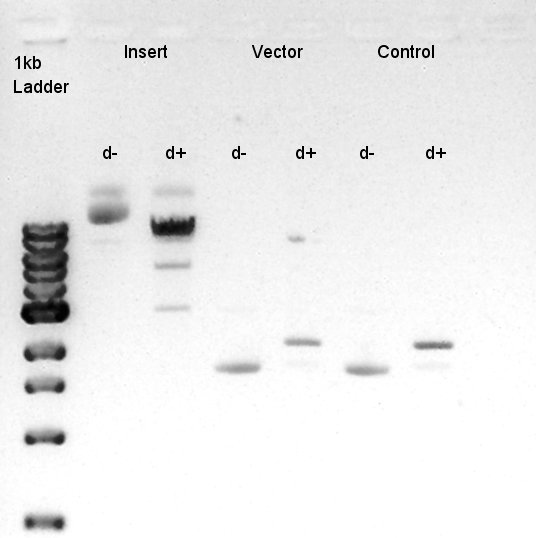
[[image:HokkaidoU2012 120914 Digestion Insert Vector.jpg|thumb|digestion result]] | [[image:HokkaidoU2012 120914 Digestion Insert Vector.jpg|thumb|digestion result]] | ||
| - | We suppose the Insert DNA (RBS-phaB-RBS-eYFP-dT) has contained Vector-dimer DNA as contamination. But desired DNA fragment (about 1600 bp) bond also appeared in the result of migration. We picked up the fragment and extracted from | + | We suppose the Insert DNA (RBS-phaB-RBS-eYFP-dT) has contained Vector-dimer DNA as contamination. But desired DNA fragment (about 1600 bp) bond also appeared in the result of migration. We picked up the fragment and extracted from gel. |
</p> | </p> | ||
Revision as of 09:55, 26 September 2012
September 10th
Digestion of eCFP-RBS-pSB1A2 and pBAD-RBS-pSB1A2
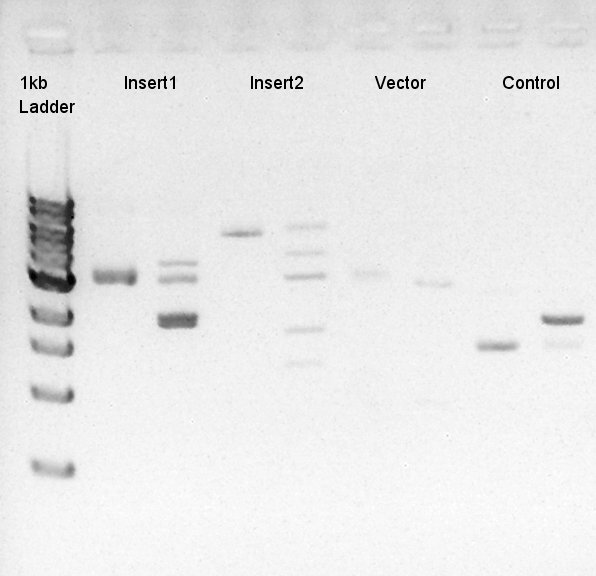
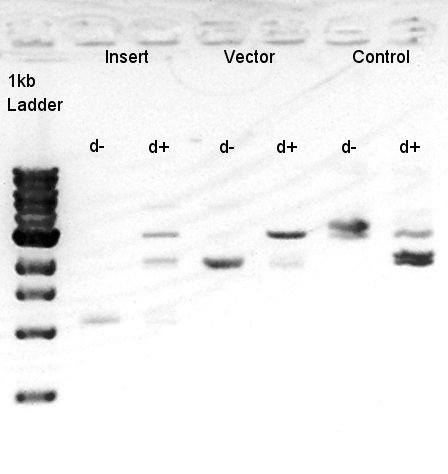
To make a construct of pBAD-RBS-eCFP-RBS-Ag43-dT-pSTV28 by 3piece ligation, we digested pBAD-RBS-eCFP-RBS-pSB1A2 with EcoRI & SpeI, Ag43-dT-pSB1AK3 (previously digested with HindIII) with XbaI & NotI and pSTV28 with EcoRI and NotI. Then we digested pT7-RBS-pSB1C3 with XbaI & SpeI as a control for confirmation of the ability of restriction enzyme. Insert1 (pBAD-RBS-eCFP-RBS-pSB1A2)
| DNA solution ( 35ng/ul) | 17 ul |
| EcoRI | 1 ul |
| SpeI | 1 ul |
| 10xH buffer | 3 ul |
| DW | 8 ul |
| Total | 30 ul |
Insert2 (Ag43-dT-pSB1AK3)
| DNA solution ( 35ng/ul) | 25 ul |
| XbaI | 1 ul |
| NotI | 1 ul |
| 10xK buffer | 1.5 ul |
| 100xBSA | 0.3ul |
| DW | 1.5 ul |
| Total | 30 ul |
Vector(pSTV28)
| DNA solution ( 15ng/ul) | 9 ul |
| EcoRI | 1 ul |
| NotI | 1 ul |
| 10xH buffer | 2 ul |
| 10xBSA | 0.2ul |
| DW | 7 ul |
| Total | 20 ul |
control (pT7-RBS-pSB1C3)
| DNA solution (30~40 ng/ul) | 10 ul |
| XbaI | 1 ul |
| SpeI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 6 ul |
| Total | 20 ul |
| Number | Degree | Minute |
| 1 | 37 | 120 |
| 2 | 70 | 20 |
| 3 | 4 | HOLD |
Ethanol precipitation of ptet-RBS-eYFP-dT-pSB1A2 and pSTV28
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged at 14000 rpm, 30 min at 4C.
- Remove supernatant and added 220 ul of 70% ethanol.
- Centrifuged at 15000 rpm, 15 min at 4C.
- Remove supernatant and air drying at room temperature then added 5 ul of DW.
We confirmed that the concentration of Insert1 DNA solution is 50 ng/ul, Insert2 DNA solution is 10~15 ng/ul and Vector DNA solution is 10 ng/ul.
Ligation of pBAD-RBS-eCFP-RBS, Ag43-dT and pSTV28
| Vector DNA (10 ng/ul) | 4 ul |
| Insert1 DNA (50 ng/ul) | 2 ul |
| Insert2 DNA (10~15 ng/ul) | 4 ul |
| Ligation Mighty Mix | 10 ul |
| Total | 20 ul |
Ligation reaction time was in detail below.
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
Transformation of pBAD-RBS-eCFP-RBS-Ag43-dT-pSTV28
Transformation of DH5α.
- Mixed 2 ul pBAD-RBS-eCFP-RBS-Ag43-dT-pSTV28 ligation product to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30 min.
- Mixed 350 ul of LB.
- Incubated for 2 hrs to get the resistance to Chloramphenicol.
- Prepared and Labeled two plastic plates with LB plate medium which contained appropriate antibiotics (LBC).
- Plated 300 ul of the culture onto first dish and spread.
- Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread.
- Incubated the plates at 37C for 14 hours.
September 11th
Colony PCR of pBAD-RBS-eCFP-RBS-pSB1A2
| DNA solution | 4 ul |
| Kapa-Taq(Taq polymerase) | 5 ul |
| Forward Primer(pbad-f2 primer) | 0.5 ul |
| Reverse Primer(PS-R down primer) | 0.5 ul |
| Total | 10 ul |
| Number | Degree | Second |
| 1 | 95 | 120 |
| 2 | 95 | 30 |
| 3 | 53.3 | 30 |
| 4 | 72 | 60 |
| 5 | 72 | 60 |
| 6 | 4 | HOLD |
Cycle:2~4 x 35
- We noticed that this step was actually 68.9 degree after reaction.
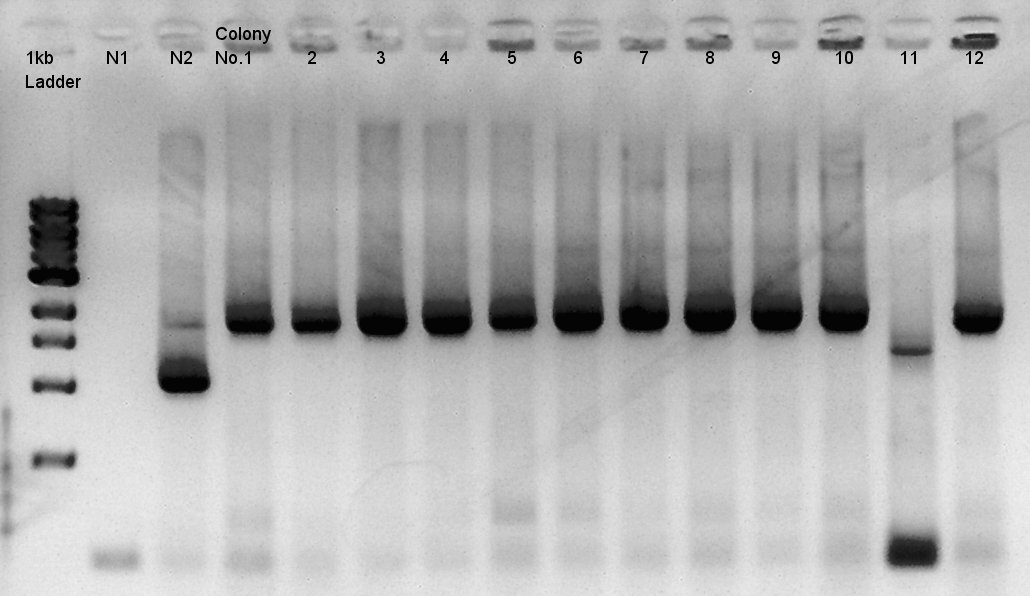
We used N1 (DW only) and N2(pBAD-RBS-Ag43-dT-pSB1A2)as controls. Desired product is about 542bp.
Because of mis-setting the PCR program, we failed to amplify desired DNA fragment so we retried the colony PCR in same reagent, correct PCR machine program.
We noticed that the ligated DNA contains at least Ag43-dT-BioBrick_Suffix complex. We selected No.2,4 for incubation for mini-prep and No.5,6 for Aggregation check (see details below).
September 12th
Analysis nucleotide sequence
We analyzed the nucleotide sequence of pBAD-RBS-Ag43-dT on pSB1AK3 and pSB1C3. We used these 6 kinds of primers. 100b up EX-F primer, pBAD-f1 primer, pBAD-f2 primer, Ag43-f1 primer, Ag43-f2 primer, Ag43-f3 primer, Ag43-f4 primer, 200b down PS-R primer
Aggregation check of pBAD-RBS-eCFP-RBS-Ag43-dT-pSTV28
Aggregation check for No.5,6 colonies selected by colony PCR at 11th.
- Prepared 5 ml LBA into glass tubes.
- Re-suspended 2 colony mixture (No.2 and No.5 respectively).
- Incubated at 37C for hrs.
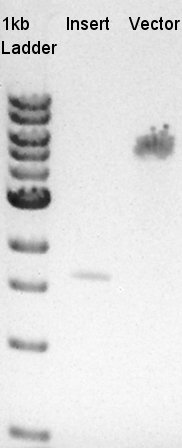
Digestion of RBS-phaB-pSB1A2 as Vector and RBS-eYFP-dT as Insert
To make a construct of RBS-phaB-RBS-eYFP-dT-pSB1A2, we digested RBS-phaB-pSB1A2 with SpeI & PstI, RBS-eYFP-dT with XbaI & PstI.
Insert (RBS-eYFP-dT)
| DNA solution ( 40ng/ul) | 25 ul |
| XbaI | 1 ul |
| NotI | 1 ul |
| 10xK buffer | 1.5 ul |
| 100xBSA | 0.3ul |
| DW | 1.5 ul |
| Total | 30 ul |
Vector(RBS-phaB-pSB1A2)
| DNA solution ( 35ng/ul) | 6 ul |
| SpeI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 10 ul |
| Total | 20 ul |
| Number | Degree | Minute |
| 1 | 37 | 120 |
| 2 | 60 | 15 |
| 3 | 4 | HOLD |
</p>
Ethanol precipitation of ptet-RBS-eYFP-dT-pSB1A2 and pSTV28
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged at 15000 rpm, 15 min at 4C.
- Remove supernatant and added 220 ul of 70% ethanol.
- Centrifuged at 15000 rpm, 10 min at 4C.
- Remove supernatant and air drying at room temperature then added 10 ul of DW.
We confirmed that the concentration of Insert DNA solution is 40 ng/ul and Vector DNA solution is 30 ng/ul.
Ligation of pBAD-RBS-eCFP-RBS, Ag43-dT and pSTV28
| Vector DNA (40 ng/ul) | 3 ul |
| Insert DNA (30 ng/ul) | 3 ul |
| Ligation Mighty Mix | 6 ul |
| Total | 12 ul |
Ligation reaction time was in detail below.
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
September 13th
Transformation of RBS-phaB-RBS-eYFP-dT-pSB1A2
Transformation of JM109.
- Mixed 2 ul ligation product to 50 ul of thawed competent cells (JM109) on ice.
- Incubated on ice for 30 min.
- Mixed 350 ul of LB.
- Prepared and Labeled two plastic plates with LB plate medium which contained appropriate antibiotics (LBA).
- Plated 300 ul of the culture onto first dish and spread.
- Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread.
- Incubated the plates at 37C for 14 hours.
Aggregation Check of pBAD-RBS-eCFP-RBS-Ag43-dT-pSTV28
- Mixed 25 ul Arabinose solution (20 %) to 5 ml LBC in glass tube.
- Incubated for 24 hrs at 37C.
Colony PCR of RBS-phaB-RBS-eYFP-dT-pSB1A2
| DNA solution | 4 ul |
| Kapa-Taq(Taq polymerase) | 10 ul |
| Forward Primer(pbad-f2 primer: 10 ng/ul) | 0.8 ul |
| Reverse Primer(PS-R down primer: 10 ng/ul) | 0.8 ul |
| DW | 4.4 ul |
| Total | 20 ul |
| Number | Degree | Second |
| 1 | 95 | 120 |
| 2 | 95 | 30 |
| 3 | 64.4 | 30 |
| 4 | 72 | 180 |
| 5 | 72 | 120 |
| 6 | 4 | HOLD |
Cycle:2~4 x 35
We used N1 (DW only) and N2(RBS-phaB-pSB1A2)as controls.
Desired product is about 1800~2000bp.
We noticed the ligated DNA contains phaB-something-SP sequence and the length is about 1600~1800bp, at least over 1000 bp. We selected No.1,2 for incubation for mini-prep and No.3,4 for stock at 4C.
Incubation of RBS-phaB-RBS-eYFP-dT-pSB1A2 for mini-prep
- Prepared 2 ml LBC into culture tubes.
- Re-suspended 2 colony mixture (No.1 and No.2 respectively).
- Incubated at 37C for 14 hrs.
September 14th
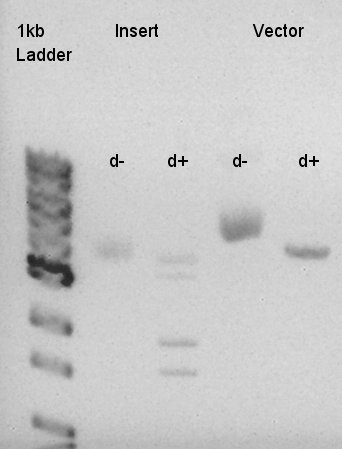
Digestion of eCFP-RBS-pSB1A2 and pBAD-RBS-pSB1A2
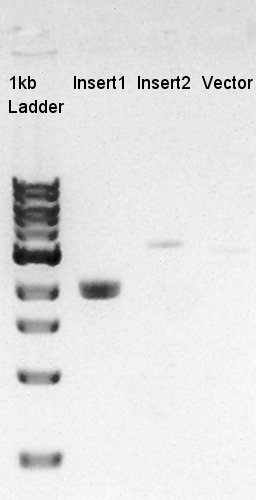
To make a construct of RBS-phaC-RBS-phaA-RBS-phaB-RBS-dT-pSB1A2, we digested RBS-phaC-RBS-phaA with SpeI & PstI, RBS-phaB-RBS-eYFP-pSB1A2 with XbaI & PstI. Insert (RBS-phaB-RBS-eYFP-dT)
| DNA solution ( 30ng/ul) | 21 ul |
| XbaI | 1 ul |
| PstI | 1 ul |
| 10xM buffer | 3 ul |
| DW | 4 ul |
| Total | 30 ul |
Vector(RBS-phaC-RBS-phaA-pSB1A2)
| DNA solution (about 30~40 ng/ul) | 6 ul |
| SpeI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 10 ul |
| Total | 20 ul |
| Number | Degree | Minute |
| 1 | 37 | 180 |
| 2 | 60 | 15 |
| 3 | 4 | HOLD |
| Number | Degree | Minute |
| 1 | 37 | 120 |
| 2 | 70 | 20 |
| 3 | 4 | HOLD |
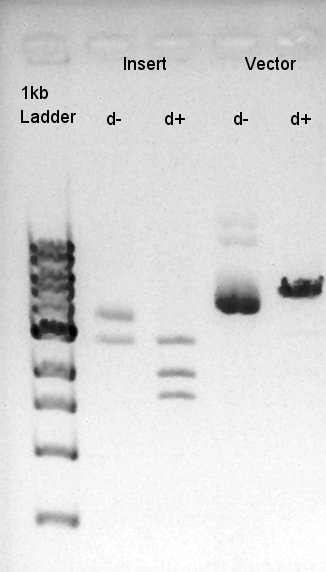
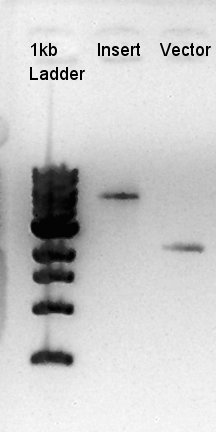
We suppose the Insert DNA (RBS-phaB-RBS-eYFP-dT) has contained Vector-dimer DNA as contamination. But desired DNA fragment (about 1600 bp) bond also appeared in the result of migration. We picked up the fragment and extracted from gel.
Ethanol precipitation of RBS-phaB-RBS-eYFP-dT and RBS-phaC-RBS-phaA-pSB1A2
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged in 14000 rpm, 30 min at 4C.
- Remove supernatant and added 220 ul of 70% ethanol.
- Centrifuged in 15000 rpm, 15 min at 4C.
- Remove supernatant and air drying in room temperature then added 5 ul of DW.
We confirmed that the concentration of Insert DNA solution is 20 ng/ul and Vector DNA solution is about 50~60 ng/ul.
Ligation of RBS-phaB-RBS-eYFP-dT and RBS-phaC-RBS-phaA-pSB1A2
| Insert DNA (20 ng/ul) | 4 ul |
| Vector DNA (50~60 ng/ul) | 1.5 ul |
| Ligation Mighty Mix | 6 ul |
| DW | 0.5 ul |
| Total | 20 ul |
Ligation reaction time was in detail below.
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
Transformation of RBS-phaC-RBS-phaA-RBS-phaB-RBS-eYFP-dT-pSB1A2
Transformation of JM109.
- Mixed 2 ul RBS-phaC-RBS-phaA-RBS-phaB-RBS-eYFP-dT-pSB1A2 ligation product to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30 min.
- Mixed 350 ul of LB.
- Prepared and Labeled two plastic plates with LB plate medium which contained appropriate antibiotics (LBA).
- Plated 300 ul of the culture onto first dish and spread.
- Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread.
- Incubated the plates at 37C for 13 hours.
September 15th
Digestion of eCFP-RBS-pSB1A2 and pBAD-RBS-pSB1A2
To make a construct of RBS-phaC-RBS-phaA-RBS-phaB-RBS-dT-pSB1A2, we digested RBS-phaC-RBS-phaA with SpeI & PstI, RBS-phaB-RBS-eYFP-pSB1A2 with XbaI & PstI. Insert (RBS-phaB-RBS-eYFP-dT)
| DNA solution ( 30ng/ul) | 21 ul |
| XbaI | 1 ul |
| PstI | 1 ul |
| 10xM buffer | 3 ul |
| DW | 4 ul |
| Total | 30 ul |
Vector(RBS-phaC-RBS-phaA-pSB1A2)
| DNA solution (about 30~40 ng/ul) | 6 ul |
| SpeI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 10 ul |
| Total | 20 ul |
| Number | Degree | Minute |
| 1 | 37 | 120 |
| 2 | 60 | 15 |
| 3 | 4 | HOLD |
| Number | Degree | Minute |
| 1 | 37 | 120 |
| 2 | 70 | 20 |
| 3 | 4 | HOLD |
Ethanol precipitation of RBS-phaB-RBS-eYFP-dT and RBS-phaC-RBS-phaA-pSB1A2
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged in 14000 rpm, 30 min at 4C.
- Remove supernatant and added 220 ul of 70% ethanol.
- Centrifuged in 15000 rpm, 15 min at 4C.
- Remove supernatant and air drying in room temperature then added 5 ul of DW.
We confirmed that the concentration of Insert DNA solution is 20 ng/ul and Vector DNA solution is about 50~60 ng/ul.
Ligation of RBS-phaB-RBS-eYFP-dT and RBS-phaC-RBS-phaA-pSB1A2
| Insert DNA (20 ng/ul) | 4 ul |
| Vector DNA (50~60 ng/ul) | 1.5 ul |
| Ligation Mighty Mix | 6 ul |
| DW | 0.5 ul |
| Total | 20 ul |
Ligation reaction time was in detail below.
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
Transformation of RBS-phaC-RBS-phaA-RBS-phaB-RBS-eYFP-dT-pSB1A2
Transformation of JM109.
- Mixed 2 ul RBS-phaC-RBS-phaA-RBS-phaB-RBS-eYFP-dT-pSB1A2 ligation product to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30 min.
- Mixed 350 ul of LB.
- Prepared and Labeled two plastic plates with LB plate medium which contained appropriate antibiotics (LBA).
- Plated 300 ul of the culture onto first dish and spread.
- Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread.
- Incubated the plates at 37C for 17 hours.
September 16th
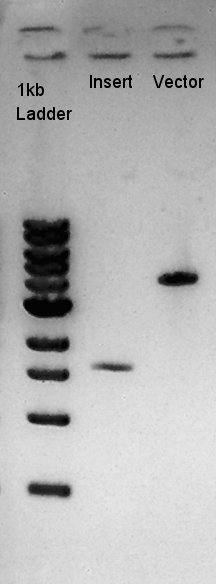
Digestion of pBAD-RBS-eCFP-RBS-Ag43-dT-pSTV28 and pT7-RBS-pSB1C3
To make a construct of pBAD-RBS-eCFP-RBS-Ag43-dT-pSB1C3, we digested pBAD-RBS-eCFP-RBS-Ag43-dT-pSTV28 with EcoRI & SpeI, and pT7-RBS-pSB1C3 with EcoRI & SpeI. As a control, we digested pT7-RBS-pSB1C3 with only EcoRI to confirm the digestibility of This DNA (The digestibility with SpeI has confirmed in August 26th). Insert (pBAD-RBS-eCFP-RBS-Ag43-dT-pSTV28)
| DNA solution (about 35ng/ul) | 41 ul |
| EcoRI | 1 ul |
| SpeI | 1 ul |
| 10xH buffer | 5 ul |
| DW | 2 ul |
| Total | 50 ul |
Vector(pT7-RBS-pSB1C3)
| DNA solution (about 30~40 ng/ul) | 2 ul |
| EcoRI | 1 ul |
| SpeI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 14 ul |
| Total | 20 ul |
control (pT7-RBS-pSB1C3)
| DNA solution (about 30~40 ng/ul) | 2 ul |
| EcoRI | 1 ul |
| 10xH buffer | 1 ul |
| DW | 6 ul |
| Total | 10 ul |
| Number | Degree | Minute |
| 1 | 37 | 120 |
| 2 | 60 | 15 |
| 3 | 4 | HOLD |
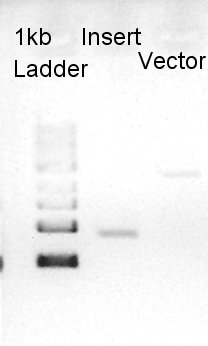
We confirmed 4 bonds in the result of Inset DNA digestion. A Bond which shows the highest concentration and places about 8k ~ 10k bp area would be pBAD-RBS-eCFP-RBS-Ag43-pSTV28 construct. We considered about it bond whether remained from digestion, or at first formed dimer and separated by digestion. In either case, our target product has about 5200 bp and there is appropriate bond. We extracted the target bond from TBE gel and went next step.
Ethanol precipitation of pBAD-RBS-eCFP-RBS-Ag43-dT--pSB1C3
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged in 15000 rpm, 15 min at 4C.
- Remove supernatant and added 220 ul of 70% ethanol.
- Centrifuged in 15000 rpm, 10 min at 4C.
- Remove supernatant and air drying in room temperature then added 5 ul of DW.
Ligation of pBAD-RBS-eCFP-RBS-Ag43-dT and pSB1C3
| Insert DNA | 4 ul |
| Vector DNA | 1 ul |
| Ligation Mighty Mix | 5 ul |
| Total | 10 ul |
Ligation reaction time was in detail below.
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
Transformation of pBAD-RBS-eCFP-RBS-Ag43-dT-pSB1C3
Transformation of DH5α.
- Mixed 2 ul pBAD-RBS-eCFP-RBS-Ag43-dT--pSB1C3 ligation product to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30 min.
- Mixed 350 ul of LB.
- Incubated for 2 hrs to get the resistance to Chloramphenicol.
- Prepared and Labeled two plastic plates with LB plate medium which contained appropriate antibiotics (LBC).
- Plated 300 ul of the culture onto first dish and spread.
- Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread.
- Incubated the plates at 37C for 13 hours.
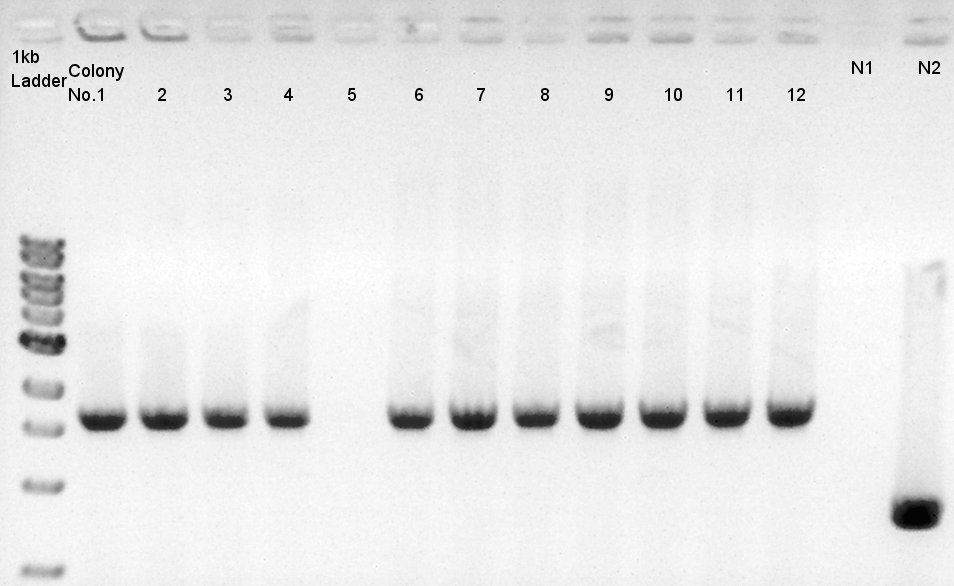
Colony PCR of RBS-phaC-RBs-phaA-RBS-phaB-RBS-eYFP-dT-pSB1A2
| DNA solution | 4 ul |
| Kapa-Taq(Taq polymerase) | 10 ul |
| Forward Primer(phaA-1083bp-F primer: 10 ng/ul) | 0.8 ul |
| Reverse Primer(PS-R down primer: 10 ng/ul) | 0.8 ul |
| DW | 4.4 ul |
| Total | 20 ul |
| Number | Degree | Second |
| 1 | 95 | 120 |
| 2 | 95 | 30 |
| 3 | 68.9 | 30 |
| 4 | 72 | 180 |
| 5 | 72 | 120 |
| 6 | 4 | HOLD |
Cycle:2~4 x 35
We used N1 (DW only) and N2(RBS-phaC-RBS-phaA-RBS-phaB-dT-pSB1A2)as controls.
Desired product is about 1800~2000bp.
We noticed the ligated DNA contains phaB-something-SP sequence and the length is about 1600~1800bp, at least over 1000 bp. We selected No.1,2 for incubation for mini-prep and No.3,4 for stock at 4C.
Incubation of RBS-phaB-RBS-eYFP-dT-pSB1A2 for mini-prep
- Prepared 2 ml LBA into culture tubes.
- Re-suspended 2 colony mixture (No.1 and No.2 respectively).
- Incubated at 37C for hrs.
 "
"