Team:Bielefeld-Germany/Labjournal/week16
From 2012.igem.org
(Difference between revisions)
(→Saturday August 18th) |
|||
| Line 1: | Line 1: | ||
{{Team:Bielefeld/Head}} | {{Team:Bielefeld/Head}} | ||
| + | |||
| + | |||
| + | <html> | ||
| + | <style type="text/css"> | ||
| + | |||
| + | ul {list-style-image:none;} | ||
| + | #bodyContent{ | ||
| + | background-color: white; | ||
| + | } | ||
| + | |||
| + | </style> | ||
| + | |||
| + | <!-- navigator --> | ||
| + | <div id="nav" class="tabs"> | ||
| + | <div class="scroller"> | ||
| + | <ul style="list-style-type:none"> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/Starting"><strong>Prologue</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week1"><strong>Week 1</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week2"><strong>Week 2</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week3"><strong>Week 3</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week4"><strong>Week 4</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week5"><strong>Week 5</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week6"><strong>Week 6</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week7"><strong>Week 7</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week8"><strong>Week 8</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week9"><strong>Week 9</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week10"><strong>Week 10</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week11"><strong>Week 11</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week12"><strong>Week 12</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week13"><strong>Week 13</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week14"><strong>Week 14</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week15"><strong>Week 15</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week16"><strong>Week 16</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week17"><strong>Week 17</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week18"><strong>Week 18</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week19"><strong>Week 19</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week20"><strong>Week 20</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week21"><strong>Week 21</strong></a></li> | ||
| + | <li><a href="https://2012.igem.org/Team:Bielefeld-Germany/Labjournal/week22"><strong>Week 22</strong></a></li> | ||
| + | |||
| + | </ul> | ||
| + | |||
| + | </div> | ||
| + | </div> | ||
| + | </html> | ||
<div style="text-align:justify;"> | <div style="text-align:justify;"> | ||
Revision as of 10:11, 23 September 2012
Contents |
Week 16 (08/13 - 08/19/12)
Monday August 13th
- Team Cultivation & Purification:
- Today we discussed that we may get activity if we start to purify our laccases. So from now we will purify our products before measuring them. We hope this will bring promising results. Therefore we searched for a useful method.
- Team Site Directed Mutagenesis:
- Gradient-PCR of Tvel10 55 to 66°C with DMSO (12 Steps) resulted in a lot of product at 55°C and 63°C to 66°C; a little product at 56°-59° and 61°-62°C and no product at 60°C (59°C was the temperature Clonemanager predicted and I used before). Merged the products and cleaned them up.
- Digested the product with EcoRI, PstI and DpnI.
- Team Cellulose Binding Domain:
- We did another PCR on the <partinfo>I13522</partinfo> with the [http://partsregistry.org/Part:BBa_K863121 GFP_His]-primers with positive result.
- Plasmid-isolation of two positive clones of [http://partsregistry.org/Part:BBa_K863112 CBDclos(T7)] followed by digestion with NotI that confirmed that the plasmid is [http://partsregistry.org/Part:BBa_K863112 CBDclos(T7)]. Prepared both for sequencing.
- Plated three positve [http://partsregistry.org/Part:BBa_K863102 CBDcex(T7)]-colonies.
- Restriction of the [http://partsregistry.org/Part:BBa_K863121 GFP_His]-PCR-product with EcoRI and PstI to bring it into the pSB1C3-backbone.
- Also: Restriction of the [http://partsregistry.org/Part:BBa_K863102 CBDcex(T7)]-PCR-product with NgoMIV and PstI for an assembly.
- Restriktion of the [http://partsregistry.org/Part:BBa_K863102 CBDcex(T7)]-PCR-product with AgeI and EcoRI for the assembly.
- All digestions were done with DpnI to get rid of the templates.
- Ligation of [http://partsregistry.org/Part:BBa_K863121 GFP_His] with the pSB1C3-backbone.
- Ligation of [http://partsregistry.org/Part:BBa_K863103 CBDcex(T7) and GFP_His] with the pSB1C3-backbone.
- Transformed all ligations into KRX.
Tuesday August 14th
- Team Fungal and Plant Laccase: Today our cDNA took a bath in ethanol and got cleaned. We are pretty sure our next PCR will be a lot more successful. Check protocols for further information about the cDNA washing via ethanol precipitation.
- Team Cultivation & Purification:
- Made preculture of E. coli KRX without plasmid and with [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863020 BBa_K863020], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863000 BBa_K863000], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863005 BBa_K863005], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863010 BBa_K863010] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863015 BBa_K863015] as well as [http://partsregistry.org/Part:BBa_K525710 BBa_K525710].
- Team Site Directed Mutagenesis:
- Ligation of tvel (PCR-product) and pSB1C3 and transformation into KRX
- Gradient-PCRs (55°C bis 72 °C) with xccl-plasmid using xccl-g3633c and xccl-g2247c primer-mixes, respectively, resulted in no product of the right size.
- Team Cellulose Binding Domain:
- Sequencing results showed, that the insertion of [http://partsregistry.org/Part:BBa_K863121 GFP_His] wasn't successful at all, [http://partsregistry.org/Part:BBa_K863102 CBDcex(T7)] had a lot of mutations and [http://partsregistry.org/Part:BBa_K863112 CBDclos(T7)] had one silent mutation.
- On the [http://partsregistry.org/Part:BBa_K863121 GFP_His]-transformation-dish did not grow any colony. Did a new transformation with a little more ligation-product and plated the cells on selection-agar.
- Plasmid-isolation of the three positive [http://partsregistry.org/Part:BBa_K863102 CBDcex(T7)]-plasmids and digestion showed not the correct fragments. Plated some more positive colonies on selection-agar for plasmid-isolation.
- The [http://partsregistry.org/Part:BBa_K863103 CBDcex(T7)+GFP_His]-dish had only nine colonies and a colony-PCR showed that none of them had the right insert.
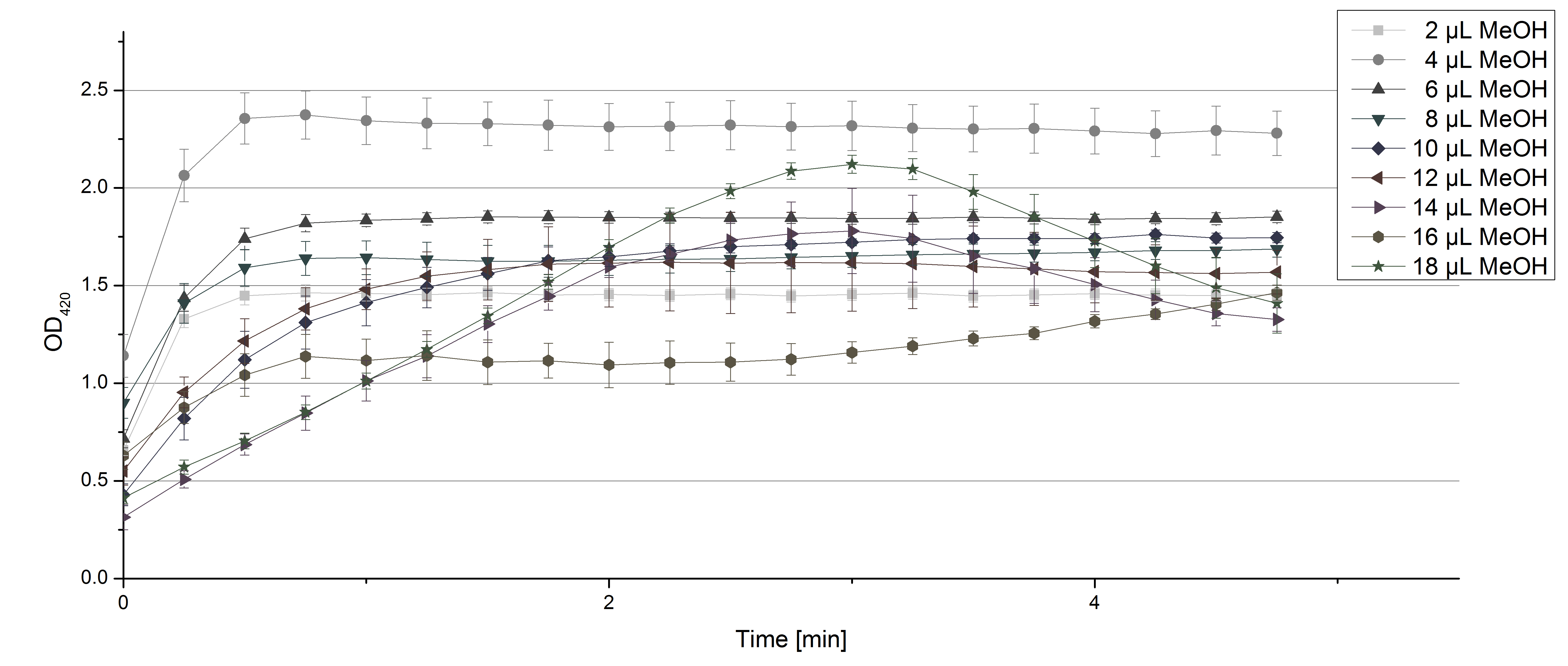
- Team Activity Tests: Again our skills in measuring laccase activity were in demand today. We had a job incoming from Team Substrate Analytic. Since they need to dissolve their substrates in methanol or acetonitrile, they were curious about laccase activity related to these solvents. And they infected us with their curiosity! So we started measurements using 0.1 U TVEL0 Laccase, 40 µL sodium acetate buffer and different amounts of MeOH or acetonitrile respectively, ad 200 µL H2O. Our tested range went from 2 µL to 18 µL. The results show that MeOH and acetonitril have an impact on the ability of TVEL0 to oxidize ABTS. Using 16 µL MeOH (which means 8% of the amount of the sample contained MeOH) led to a different saturation curve as usual. The saturation couldn't be reached any more (see Fig. 1). Acetonitrile had a noticeable impact when using 5% (14 µL) of it in relation to the sample (see Fig. 2). But in the end both solvents couldn't stop TVEL0 laccase from being active. We are happy to tell Team Substrate Analytics that they can proceed as planned and dissolve the substrates in the tested solvents.
Wednesday August 15th
- Team Wiki: We set up some more wiki rules. Today´s rules were about citations. We agreed von some standards that will for sure help make the wiki a little prettier. Have a look:
- Paper: Exampleman M et al. (2002). The example paper. The example journal (Volume): pages.
- Website: Name of website, URL, Index, date site visited
- Book: Examplewomen M et al. (1999). The example book. The example publisher (edition).
Next will be a standard for charts.
- Team Cultivation & Purification:
- Flask cutivation of E. coli KRX with [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863020 BBa_K863020], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863000 BBa_K863000],[http://partsregistry.org/wiki/index.php?title=Part:BBa_K863005 BBa_K863005], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863010 BBa_K863010] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863015 BBa_K863015]. We used E. coli KRX negative control as well as E. coli KRX with [http://partsregistry.org/Part:BBa_K525710 BBa_K525710] as positive control.
- Settings: 1 L flasks without baffles, final volume: 250 mL, autoinduction medium supplemented with 60 µg/mL chloramphenicol, 37 °C, 120 rpm, single determination
- Flask cutivation of E. coli KRX with [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863020 BBa_K863020], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863000 BBa_K863000],[http://partsregistry.org/wiki/index.php?title=Part:BBa_K863005 BBa_K863005], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863010 BBa_K863010] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863015 BBa_K863015]. We used E. coli KRX negative control as well as E. coli KRX with [http://partsregistry.org/Part:BBa_K525710 BBa_K525710] as positive control.
- Team Site Directed Mutagenesis:
- Colony-PCR of tvel10-colonies resulted in small bands. Quickly explained: tvel10 still had illegal PstI-restrictions-sites. Digestion of tvel10 PCR-product with NotI as well as digestion of RFP-pSB1C3 with NotI
- Team Cellulose Binding Domain:
- Plasmid-isolation of the three positive [http://partsregistry.org/Part:BBa_K863102 CBDcex(T7)]-colonies, but digestion showed no positive result, again.
- Colony-PCR of [http://partsregistry.org/Part:BBa_K863121 GFP_His] and [http://partsregistry.org/Part:BBa_K863103 CBDcex(T7)+GFP_His] did not show any positive result, ether.
Thursday August 16th
- Team Cultivation & Purification:
- Today we harvested the cells from the cultivation on 08/15, disrupted them in equilibration buffer via sonification, centrifugated and purified the supernatant via HisTrap column. The purified samples in 500 mM Imidazol were given to the activity test team.
- Team Site Directed Mutagenesis:
- Clean-up of tvel10 and pSB1C3 (both cut with NotI); Dephosphorilation of pSB1C3 with SAP and ligation. Transformed into KRX and plated on select-agar.
- Team Cellulose Binding Domain:
- Colony-PCR of [http://partsregistry.org/Part:BBa_K863103 CBDcex(T7)+GFP_His]-clones with no result, even the positive-control was negative, so we plated three colonies for a test-digestion instead.
- Digestion of [http://partsregistry.org/Part:BBa_K863112 CBDclos(T7)] with AgeI and PstI and digestion of the [http://partsregistry.org/Part:BBa_K863121 GFP_His]-PCR-product with NgoMIV and PstI over-night.
Friday August 17th
- Team Cellulose Binding Domain:
- Stopped digestion of [http://partsregistry.org/Part:BBa_K863112 CBDclos(T7)] and [http://partsregistry.org/Part:BBa_K863121 GFP_His]. Dephosphorylated pSB1C3+[http://partsregistry.org/Part:BBa_K863112 CBDclos(T7)].
- Plasmid-isolation of the three [http://partsregistry.org/Part:BBa_K863103 CBDcex(T7)+GFP_His]-dishes.
- The sequencing of the two [http://partsregistry.org/Part:BBa_K863112 CBDclos(T7)]-plasmids showed the same result as the first: one silent mutation. We decided to carry on with this plasmid.
Saturday August 18th
- Team Cloning of Bacterial laccases:
- Today we performed a ligation from the digestions we have done before. The ligation included of course the T7 promotor, the backbone pSB1C3 and the individual laccase genes. Ligation was performed with T4 ligase for 20 minutes at room temperature and stopped through a five minute incubation at 70°C.
- Team Cellulose Binding Domain:
- Ligated [http://partsregistry.org/Part:BBa_K863112 CBDclos(T7)] and [http://partsregistry.org/Part:BBa_K863121 GFP_His], transform into KRX and plated on CM-selection-Agar.
Sunday August 19th
- Team Site Directed Mutagenesis:
- Colony-PCR of tvel10-colonies with three positive result.
- Plated those three on select-agar for plasmid-isolation.
- Team Cellulose Binding Domain:
- Colony-PCR of colonies from the [http://partsregistry.org/Part:BBa_K863113 CBDclos(T7)+GFP_His]-trafo-dish with only negative results.
| 55px | | | | | | | | | | |
 "
"