Team:Bielefeld-Germany/Labjournal/week9
From 2012.igem.org
(Difference between revisions)
(→Wednesday June 27th) |
(→Wednesday June 27th) |
||
| Line 14: | Line 14: | ||
===Wednesday June 27th=== | ===Wednesday June 27th=== | ||
* '''Team shuttle vector:''' Cultivation of ''Komagataella pastoris'' X33 and GS115 in YPD media for isolation of the genomic DNA. | * '''Team shuttle vector:''' Cultivation of ''Komagataella pastoris'' X33 and GS115 in YPD media for isolation of the genomic DNA. | ||
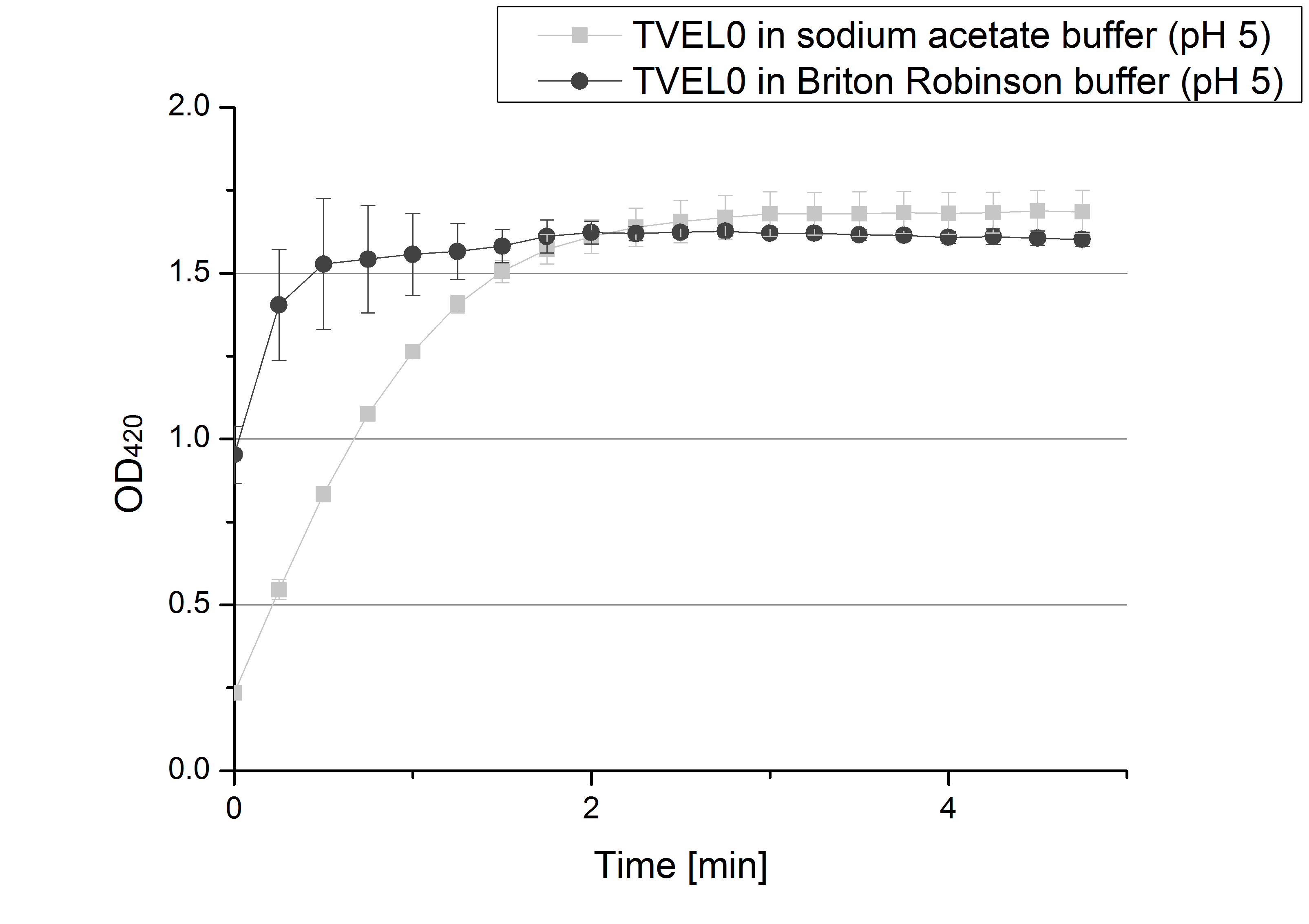
| - | [[File:Bielefeld2012 SoAc BRP comparisonTVEL0.png|thumb|right|Activity measurement of TVELO in sodium acetate buffer | + | [[File:Bielefeld2012 SoAc BRP comparisonTVEL0.png|thumb|right|Activity measurement of TVELO in sodium acetate buffer (pH5) and Briton Robinson buffer (pH 5). Measurements were taken via OD<sub>420</sub> of oxidized ABTS.]] |
* '''Team Activity Tests''': We like our new cooperation with Team Immobilization. The thing is, that they don´t like our buffer. Sodium acetate (pH 5) seems perfect for activity tests but apparently not for immobilization. What they prefer is a Briton Robinson buffer (pH 5). To find out whether there is a difference between the two buffers that causes different activity habits of our laccase TVEL0, we setup comparable measurements with the two buffers and TVEL0. We concluded that the laccase in sodium acetate buffer shows a slower saturation but all in all both laccases reach the same maximum so that it is ok for us to use both buffer systems. | * '''Team Activity Tests''': We like our new cooperation with Team Immobilization. The thing is, that they don´t like our buffer. Sodium acetate (pH 5) seems perfect for activity tests but apparently not for immobilization. What they prefer is a Briton Robinson buffer (pH 5). To find out whether there is a difference between the two buffers that causes different activity habits of our laccase TVEL0, we setup comparable measurements with the two buffers and TVEL0. We concluded that the laccase in sodium acetate buffer shows a slower saturation but all in all both laccases reach the same maximum so that it is ok for us to use both buffer systems. | ||
Revision as of 13:01, 19 September 2012
Contents |
Week 9 (06/25 - 07/01/12)
Monday June 25th
- Team Bacterial Laccases: Retried the DNA isolation from S. griseus and S. lavendulae without any success.
Tuesday June 26th
- Team A. thaliana laccase: The thing about plants is that they have to grow. Fortunately we got 6 beautiful 4 weeks-old wildtype plants from Patrick Treffon from the Institute of Plant Physiology and Biochemistry at Bielefeld University. With the help of the [http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi efp-Browser] we found out that the [http://www.ncbi.nlm.nih.gov/protein/AAM77221.1 laccase] in A. thaliana is only expressed in the developing seeds. So we now have to wait for the siliques to develop.
- Team shuttle vector: Prepare the YPD Media for cultivation of the yeast strains Komatagaella patoris X33 (wildtype) and GS115 (Invitrogen). Both organisms are provided from the [http://www.techfak.uni-bielefeld.de/ags/fermtech/ chair of Fermentation Engineering] (D5) from Dr. Thomas Hug.
Wednesday June 27th
- Team shuttle vector: Cultivation of Komagataella pastoris X33 and GS115 in YPD media for isolation of the genomic DNA.
- Team Activity Tests: We like our new cooperation with Team Immobilization. The thing is, that they don´t like our buffer. Sodium acetate (pH 5) seems perfect for activity tests but apparently not for immobilization. What they prefer is a Briton Robinson buffer (pH 5). To find out whether there is a difference between the two buffers that causes different activity habits of our laccase TVEL0, we setup comparable measurements with the two buffers and TVEL0. We concluded that the laccase in sodium acetate buffer shows a slower saturation but all in all both laccases reach the same maximum so that it is ok for us to use both buffer systems.
Thursday June 28th
- Team Wiki: Today we browsed our wiki and were not very impressed: it's a lonesome place. So we started to think of how we could blow a little more life into it. For this, texts should appear soon on our wiki. To manage this bunch of work, we divided the subtopics of our wiki and appointed them to group members. Now everyone has a topic which he is responsible for. And that includes writing the texts, uploading pictures and keeping the represented information updated. Before anybody had the chance to disappear behind his/her notebook being busy editing his own page, we had to establish our wiki rules:
- Use the standardized formatting as presented in our example page.
- Try to edit your text without using HTML code as far as possible and use wiki code instead. Useful advices when using wiki code are represented on our example page, too.
- If you want to change anything that does not belong to your scope of duties, ask kindly the person of charge and make sure he/she is fine with it.
- Make your text more understandable by using images and charts. But remember: you are only allowed to upload pictures if you own them or if they are published without licenses.
- Team shuttle vector: Isolation of genomic DNA from Komagataella pastoris GS115 (Invitrogen) and the wildtype X33 with the Wizward genomic DNA purification system Kit (Promega).
Friday June 29th
- Team Bacterial Laccases: Sequencing results showed that even with a new PCR product the same mutation occurs in B. pumilus laccase so it is probably already present on the plasmid which was sent to us. So we decided to use this plasmid.
Saturday June 30th
- Team Student Academy: We prepared a script for pupils containing background information and a protocol and wrote an abstract for the school academy program.
Sunday July 1st
Sunday
| 55px | | | | | | | | | | |
 "
"