Team:Bielefeld-Germany/Amsterdam/Labjournal
From 2012.igem.org
JSchirmacher (Talk | contribs) (→Sunday October 21st) |
JSchirmacher (Talk | contribs) (→Thursday October 25th) |
||
| Line 395: | Line 395: | ||
===Thursday October 25th=== | ===Thursday October 25th=== | ||
*'''Team Fungal and Plant Laccases:''' | *'''Team Fungal and Plant Laccases:''' | ||
| - | ** Induction of the yeast cells with 1% (v/v) methanol was done. After 4 h the cells were harvested by centrifugation at 3000xg 4°C for 5 min | + | ** Induction of the yeast cells with 1% (v/v) methanol was done. After 4 h the cells were harvested by centrifugation at 3000xg 4°C for 5 min. After adjusting buffer composition of the supernatant the activity test was done. |
* '''Team Cellulose Binding Domain:''' | * '''Team Cellulose Binding Domain:''' | ||
Revision as of 16:56, 26 October 2012
Week 24 (10/08 - 10/14/12)
Contents |
Monday October 08th
All: Day off in Amsterdam!!!
Tuesday October 09th
Wednesday October 10th
- Team Shuttle Vector:
- Ligation of pSB1C3::BBa_K863202 construct in KRX cells was done.
- Team Cellulose Binding Domain:
- Gradient-PCRs of CBDcex_Freiburg; CBDclos_Freiburg; GFP_Freiburg and ecol_Freiburg (47 °C to 67°C)
- Product:
- CBDcex_Freiburg at all temperatures
- CBDclos_Freiburg at all temperatures
- GFP_Freiburg at all temperatures
- ecol_Freiburg at 53 °C to 61°C (best at 57 to 59 °C)
- pooled fractions for gel clean up
- Product:
- Gradient-PCRs of CBDcex_Freiburg; CBDclos_Freiburg; GFP_Freiburg and ecol_Freiburg (47 °C to 67°C)
- Team Site Directed Mutagenesis:
- Ordered Primers for the SDM of the illegal XbaI restriction site in the shuttle vector
- Team Cultivation and Purification:
- Made precultures of E. coli KRX with [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863000 BBa_K863000] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863005 BBa_K863005]for 19L fermentation (500mL preculture)
Thursday October 11th
- Team Cellulose Binding Domain:
- Clean-Up of the Gel-pieces (from PCR-products)
- Restriction of PCR-products (3h):
- GFP_Freiburg:
- XbaI+PstI Tango-Buffer for Ligation
- XbaI+AgeI Tango 4xAgeI for Assembly
- CBDclos/cex:
- NgoMIV+PstI P.4+BSA 2xPstI for Assembly
- GFP_Freiburg:
- Assembly and Ligation (1h with T4):
- GFP_Freiburg + CBDcex_Freiburg + J61101
- GFP_Freiburg + CBDclos_Freiburg + J61101
- GFP_Freiburg + J61101
- Transformation in E. coli KRX (3 µL, chemical):
- GFP_Freiburg + CBDcex_Freiburg + J61101
- GFP_Freiburg + CBDclos_Freiburg + J61101
- GFP_Freiburg + J61101
- All Transformations were plated on AMP-select-agar
- Team Cultivation and Purification
- 12 L Cultivation of E. coli KRX with [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863000 BBa_K863000] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863005 BBa_K863005]
- Settings:
- 12 L Cultivation of E. coli KRX with [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863000 BBa_K863000] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863005 BBa_K863005]
Bioengineering NFL 19L fermenter, autoinduction HSG medium, 60 µg/mL chloramphenicol, 19 L, 37 °C, stirrer on cascade to hold a pO2 of 50 %, airflow: 12 NL/m, 16 hours. HSG medium was choosen to get a high biomass concentration with hope for a higher amount of laccases.
- Made precultures of E. coli Rosetta Gami 2 containing [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 BBa_K863012](09/09) and <partinfo>BBa_K863022</partinfo> (09/09) behind a constitutive promoter. (500mL preculture)
Friday October 12th
- Team Cellulose Binding Domain:
- A lot of colonies on all three dishes (J61101+GFP_Freibug;J61101+GFP_Freibug+CBDcex;J61101+GFP_Freibug+CBDclos)
- no obvious green fluorescence, not even at J61101+GFP
- Maybe the combination of J23100 and J61101 isn't strong enough to express a visible amount of GFP
- Colony-PCR of 16 colonies of every dish
- J61101 + GFP_Freiburg:
- 16/16 positive
- plated two on select-agar
- J61101 + GFP_Freiburg + CBDcex:
- 8/16 positve
- plated three on select-agar
- J61101 + GFP_Freiburg + CBDclos:
- 7/16 positive
- plated three on select-agar
- J61101 + GFP_Freiburg:
- A lot of colonies on all three dishes (J61101+GFP_Freibug;J61101+GFP_Freibug+CBDcex;J61101+GFP_Freibug+CBDclos)
- Team Shuttle Vector:
- The induction with 0.5% (v/v) methanol of P. pastoris GS115 cells with integrated BBa_K863204::GFP was started.
- Team Substrate Analysis:
- Since we have seen some new peaks after Estradiol treated with Laccases in the LC-MS, we wanted to have more degradation products to do an MS so we used a higher concentradion of Estradiol and Laccase and let them incubate for 66 hours.
- Team Cultivation and Purification
- 12L Cutlivations of E. coli KRX with [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863000 BBa_K863000] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863005 BBa_K863005] were harvested and stored at 4°C until purification.
- 12 L Cultivation of E. coli Rosetta Gami 2 containing [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 BBa_K863012](09/09) and <partinfo>BBa_K863022</partinfo> (09/09)
- Settings:
Bioengineering NFL 19L fermenter, HSG medium, 60 µg/mL chloramphenicol, 300 µg/mL ampicillin, 12 L, 37 °C, stirrer on cascade to hold a pO2 of 50 %, airflow: 12 NL/m, 22-24 hours. HSG medium was chosen to get a high biomass concentration with hope for a higher amount of laccases.
Saturday October 13th
- Team Shuttle Vector:
- The P. pastoris GS115 cells integrated with BBa_K863204::GFP were also inducted with 0.5% (v/v) methanol (100%).
- Team Cultivation and Purification
- 12L Cutlivations of E. coli Rosetta Gami 2 containing [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 BBa_K863012](09/09) and <partinfo>BBa_K863022</partinfo> (09/09) were harvested and stored at 4°C until purification.
Sunday October 14th
- Team Shuttle Vector:
- The P. pastoris GS115 cells integrated with BBa_K863204::GFP were also inducted with 0.5% (v/v) methanol (100%).
- Team Fungal and Plant Laccases:
- Team Cellulose Binding Domain:
- Tried to isolate all plates, but only got low concentrations (5 ng/µL - 20 ng/µL)
- plated eight different colonies for isolation
- Team Substrate Analysis:
- We took sample from the reaction of the 12th October.
Week 25 (10/15 - 10/21/12)
Contents |
Monday October 15th
- Team Shuttle Vector:
- There are no colonies of pBS1C3::BBa_K863202 KRX transformantion.
- The P. pastoris GS115 cells integrated with BBa_K863204::GFP were harvested and the supernatant was examined on GFP by fluorescence. But there was no GFP. The next steps are: disrupt the cells and test the supernatant on GFP. Maybe the GFP could not be secreted.
- Team Fungal and Plant Laccases:
- Yeah, the yeast cells are growing.
- Team Cellulose Binding Domain:
- Tried to isolate the eight plated, but did get low concentrations again (except one J61101+GFP_Freiburg+CBDcex_Freiburg with conc >100 ng/µL)
- cleaned up some more PCR-products (GFP_Freiburg; CBDcex_Freiburg; CBDclos_Freiburg)
- Team Substrate Analysis: We stopped the reaction from the 12th October with Methanol and gave it to Dr. Marcus Persicke to measure the degradation on LC-MS
- Team Cultivation and Purification
- Harvested Cells from 12L Cutlivations of E. coli KRX with [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863000 BBa_K863000] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863005 BBa_K863005] as well as 12 L Cultivation of E. coli Rosetta Gami 2 containing [http://partsregistry.org/wiki/index.php?title=Part:BBa_K863012 BBa_K863012](09/09) and <partinfo>BBa_K863022</partinfo> (09/09) were disrupted via high-pressure homogenizer and filtrated by [http://www.millipore.com/catalogue/module/C7493 Millipore Pellicon XL 50] with first 300 kDa to seperate all celldebris and 10 kDa to concentrate the laccases. The concentrated solutions were purified by the Ni-NTA-column.
Tuesday October 16th
- Team Activity Tests:
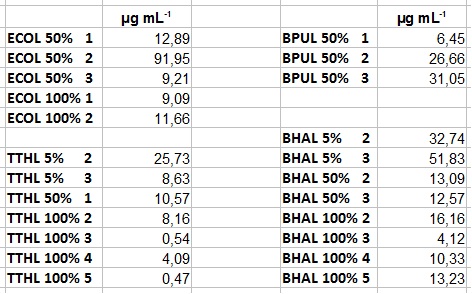
- Today we received the samples from Team Cultivation & Purification. We are really motivated to measure the activity of our new produced enzymes. But before that we have to prepare the samples. This implied the determination of the protein concentration in each fraction, which we did today. The results are listed in the following table:
- Team Cellulose Binding Domain:
- Transformed B0034 into KRX
- Transformed J23100 into KRX
- Plated both on AMP-select-agar
- Plated one more Colony of J61101 + GFP_Freiburg and J61101 + GFP_Freiburg + CBDclos
- Team Substrate Analysis: The Massspectrometry data showed two potential products after the Laccase treatment of Estradiol and Ethinyl estradiol but we unfortunatly could not identify them so we decided to do MS-MS on thoose. The products showed that after Laccase treatment both Estradiol and Ethinyl estradiol losses two H atoms which was new for us.
Wednesday October 17th
- Team Activity Tests:
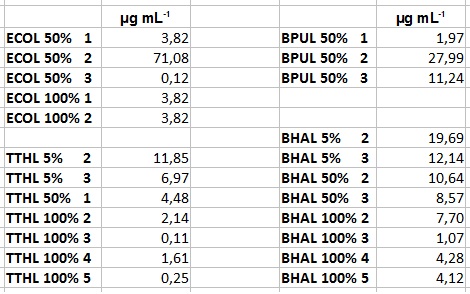
- Before starting our activity assay we re-buffered the fractions we got from Team Cultivation and Purification. It took some time, that's why we had no time for activity analysis yet. But we prepared everything for the next day, including determining the protein concentration of the fractions after they have been re-buffered. This is what we got:
- Team Shuttle Vector:
- Genomic DNA isolation of yeast cells with integrated BBa_K863204::GFP construct was done with the Kit. For genotype characterisation an PCR with the praimer pair 5AOX-Genotyp-FW and TT-Genotyp-RV was done and the fragment size was determined.
- Team Fungal and Plant Laccases:
- YPD liquid culture was inoculated with one yeast colony (BBa_K863204::TV5) and incubated at 30°C and 150 rpm.
- Team Cellulose Binding Domain:
- Picked colonies of B0034 and J23100 and plated them on AMP selection agar for plasmid isolation
- Team Site Directed Mutagenesis:
- SDM-PCR of the Shuttle vector
- Added DpnI over night
Thursday October 18th
- Team Activity Tests:
- Finally the day had come to measure the activity of our own produced laccases. This activity assay should give information about the enzyme content in every fraction. To make the measurements comparable we applied the same protein amount in every sample. Again, we used our standard activity assay protocol, but this time we used the Britton-Robinson Buffer at pH 5. Also we applied 0.1 mM ABTS and measured over night.
- Team Cellulose Binding Domain:
- Isolated J23100 plasmid
- Digested J23100 with EcoRI and PstI
- Digested pSB1C3-Backbone with EcoRI and PstI
- Clean up of pSB1C3-BB and J23100 + RFP - Insert via Gel
- Ligation of J23100 and pSB1C3-BB
- Isolated B0034 plasmid
- Digested B0034 with SpeI and PstI
- Digested GFP_Freiburg-PCR-product with XbaI and PstI
- Digested an other fraction of the GFP_Freiburg-PCR-product with XbaI and AgeI
- Assembled (Ligation):
- B0034 with GFP_Freiburg
- B0034 with GFP_Freiburg and CBDcex_Freiburg
- B0034 with GFP_Freiburg and CBDclos_Freiburg
- Transformed these three into KRX
- Team Site Directed Mutagenesis:
- Bands of 19C-PRC-product in Gel at 7 kbp (which is correct)
- Clean-Up of 19C-PRC-product
- Transformation of the 19C-PRC-product in XL1 Blue
Friday October 19th
- Team Activity Tests:
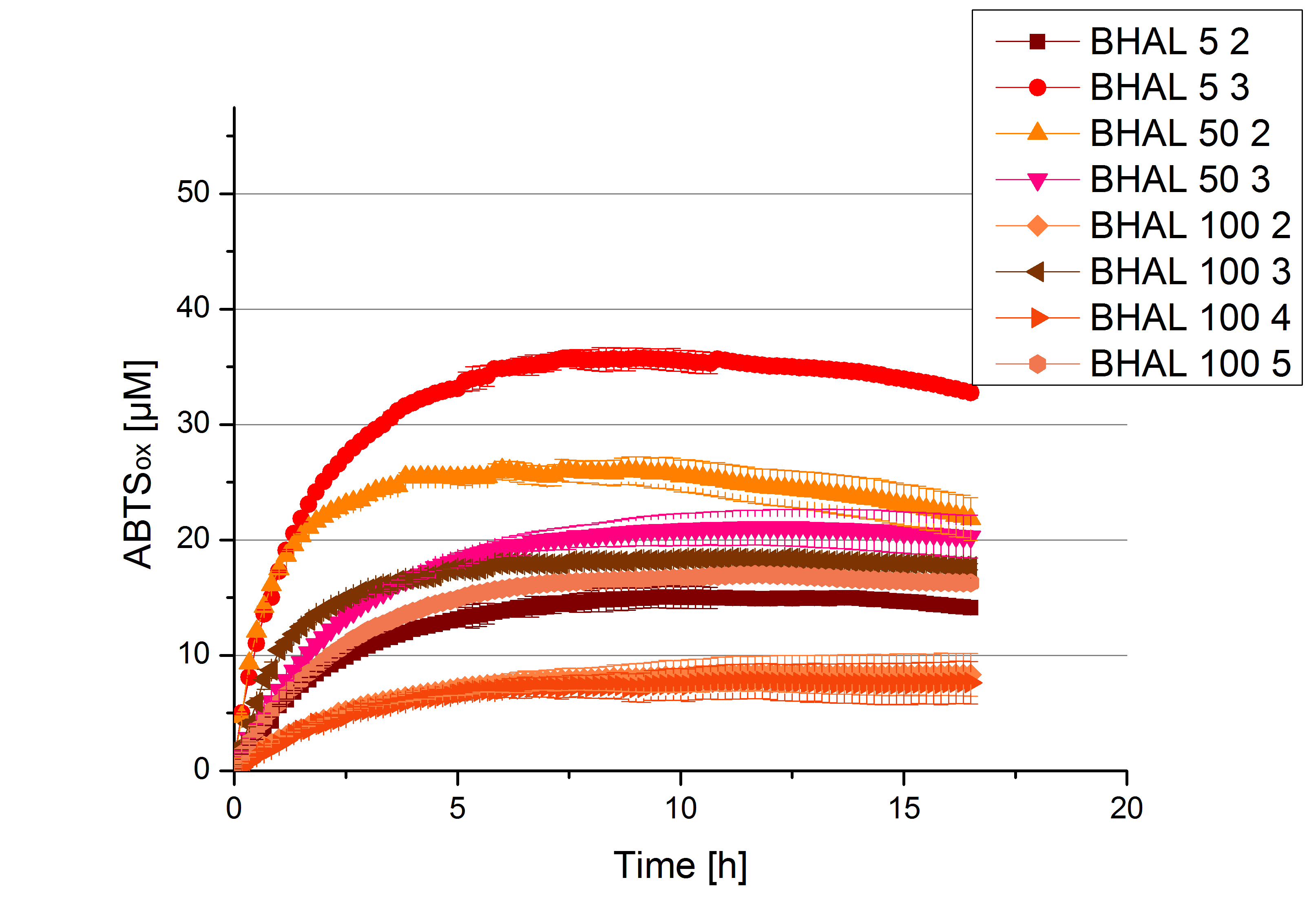
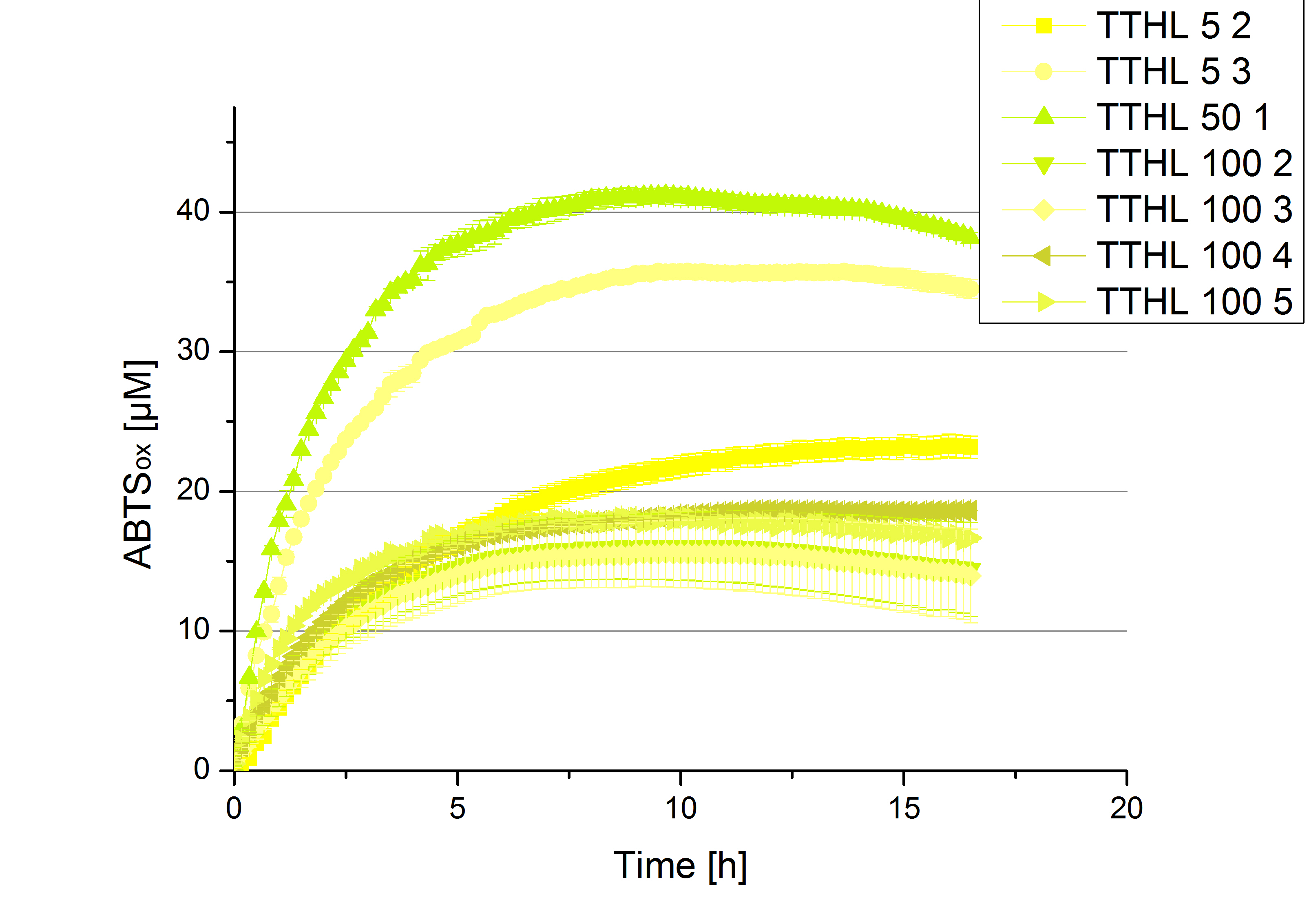
- After getting our interesting activity measurement results, we had to figure out which fraction is going to be used in the following experiments and how much laccase it contains besides other proteins. We agreed, that the most active fraction contains 90 % laccase, which is commonly used in the literature. Since we applied the same protein amount of each fraction for the activity measurements, we made sure that the results really correspond to the amount of laccase. These are the most active fractions we have chosen with their contained laccase amount:
- ECOL: Fraction 50% 2 with a laccase concentration of 63,9 µg mL-1.
- BPUL: Fraction 50% 2 with a laccase concentration of 25,1 µg mL-1.
- BHAL: Fraction 5% 3 with a laccase concentration of 10,9 µg mL-1.
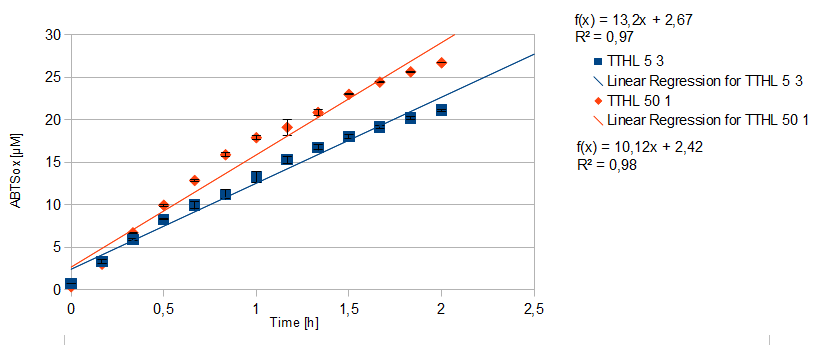
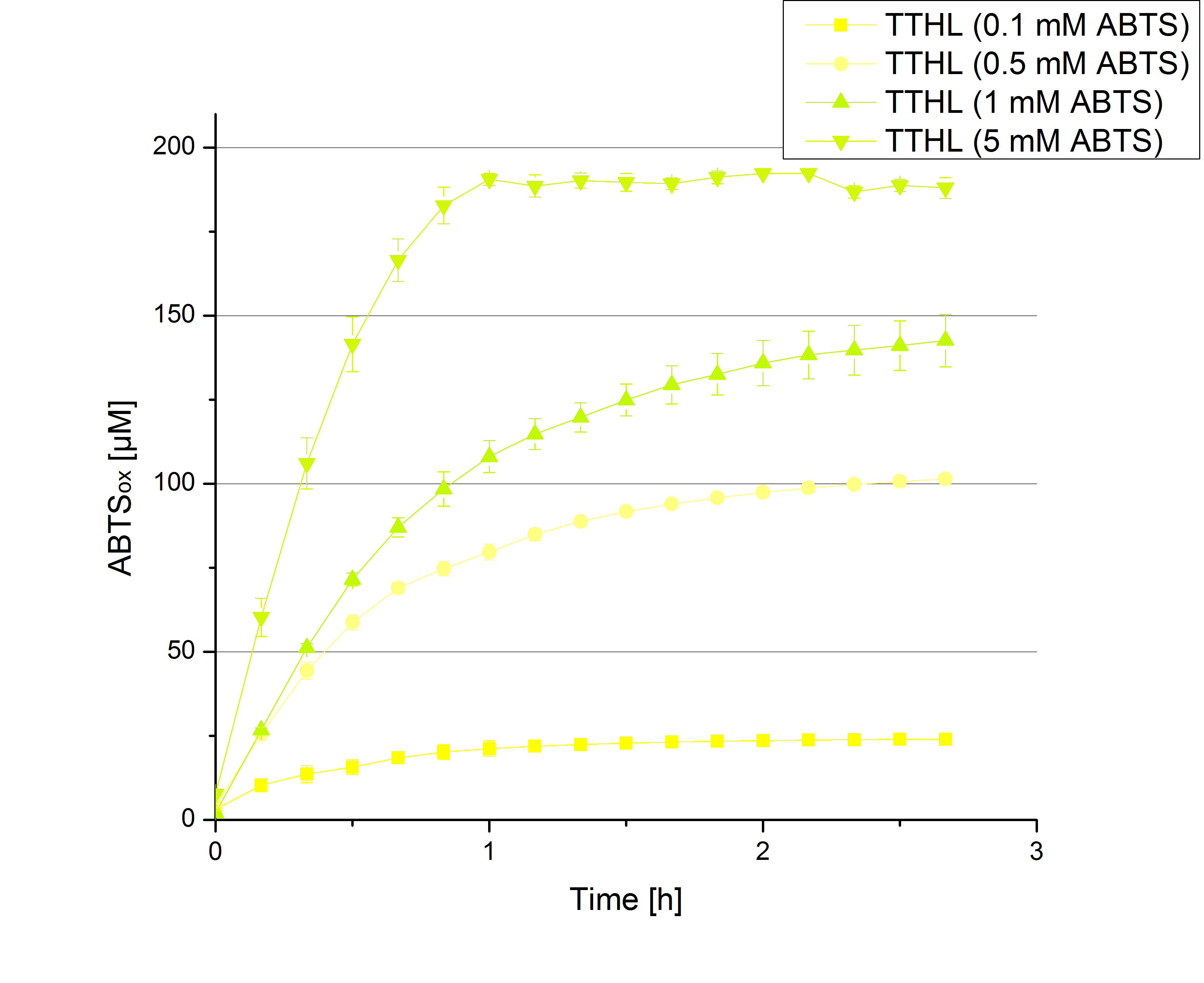
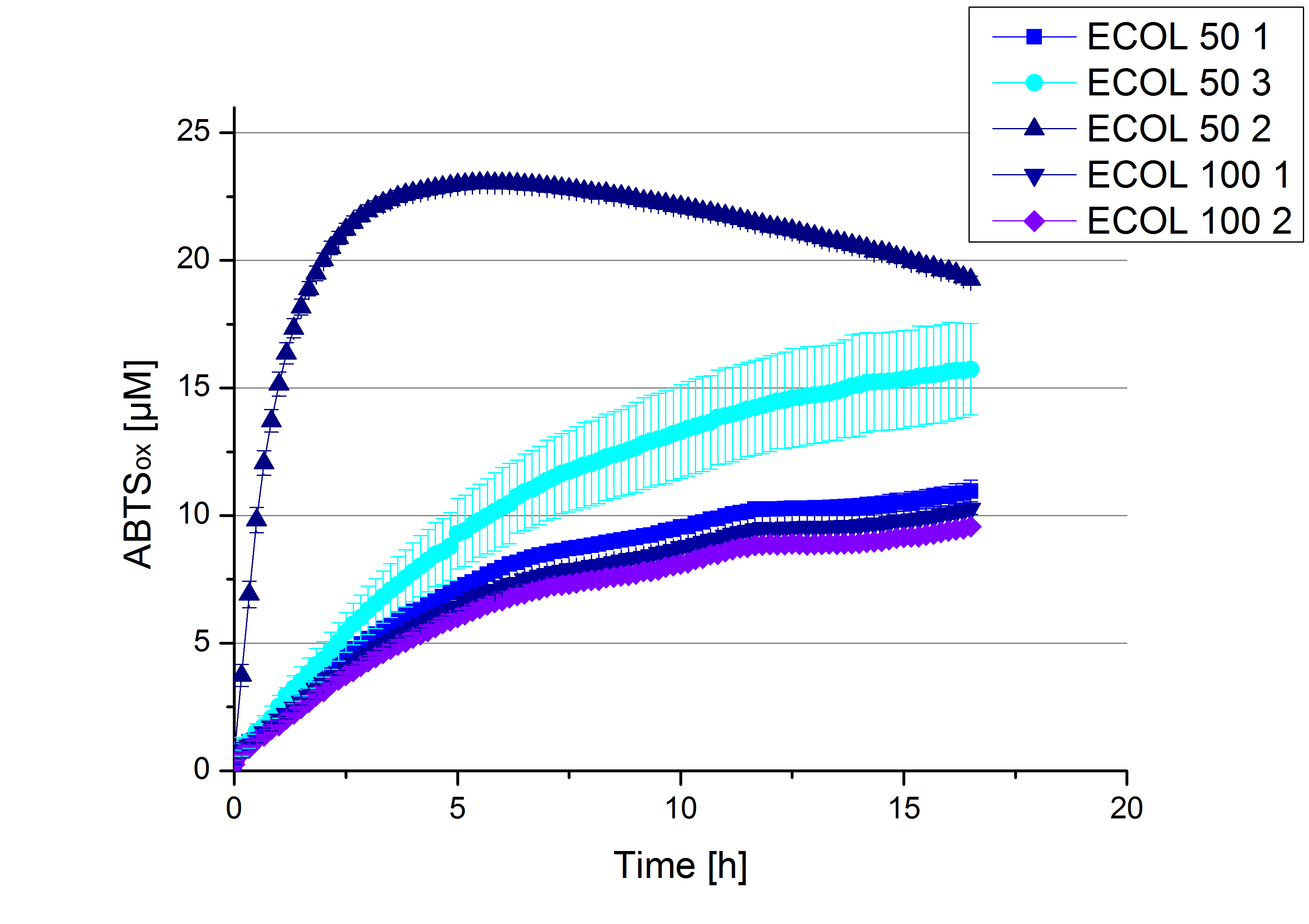
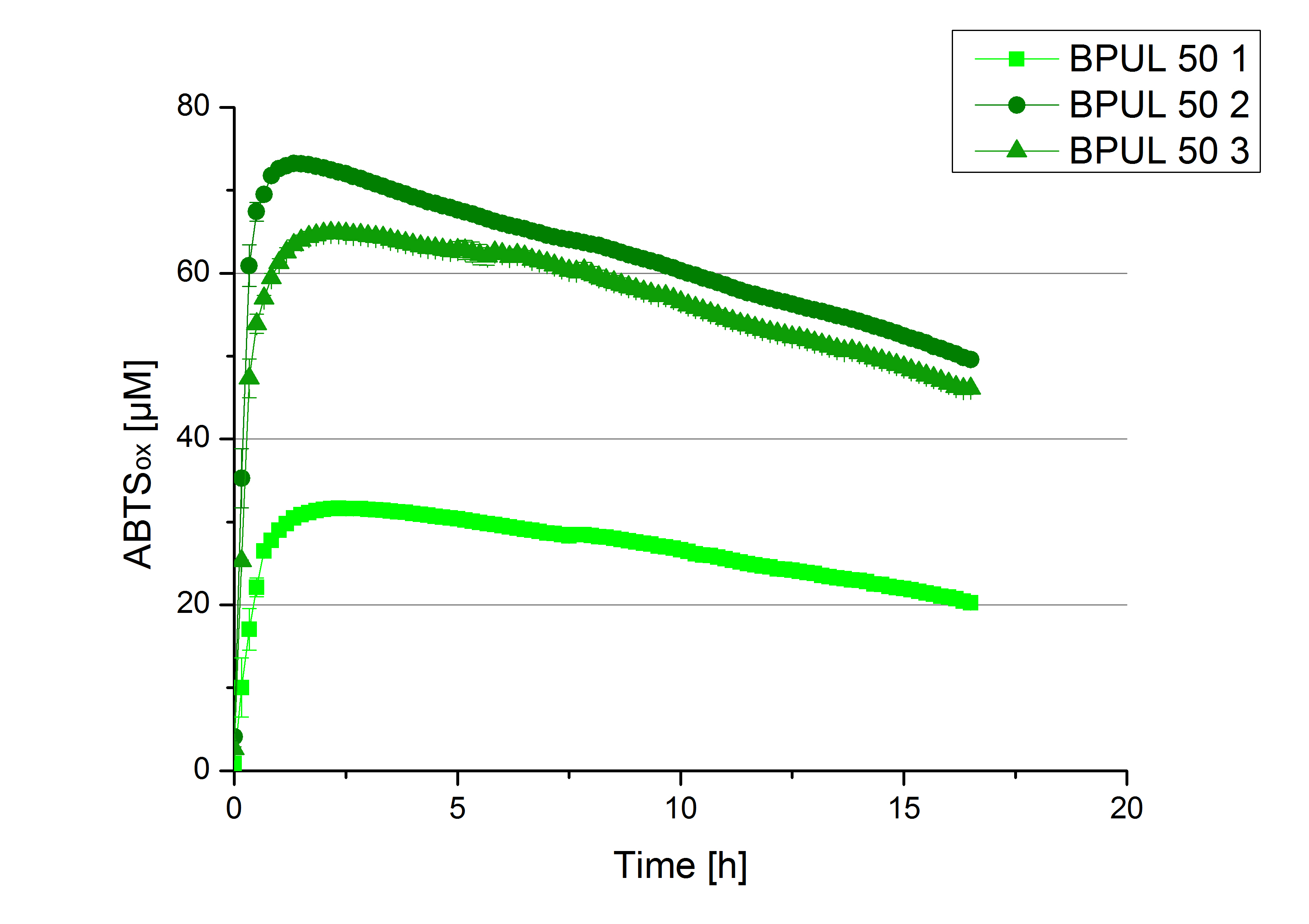
- TTHL: The best fraction of our TTHL laccase was fraction 50% 1 with a laccase concentration of 4,03 µg mL-1. In regard to Team Immobilization and Team Substrate Analysis we had to reach a higher amount of TTHL laccase. So we thought of combining two fractions. We chose the second best fraction of TTHL, which is 5% 3. To calculate the amount of contained laccase we had to compare the activity and reconsidered the slope. Since we stated the fraction with its highest activity as 90 % contained laccase, we calculated the amount of laccase regarding to that one. The following figure shows the slopes we got out of the activity from fraction 50% 1 and fraction 5% 3 (which is the second best). Accordingly to this, fraction 5% 3 contains 68,9% of laccase, which is 4,8 µg mL-1. In total, the composed fraction contains 4,4 µg mL-1.
- After getting our interesting activity measurement results, we had to figure out which fraction is going to be used in the following experiments and how much laccase it contains besides other proteins. We agreed, that the most active fraction contains 90 % laccase, which is commonly used in the literature. Since we applied the same protein amount of each fraction for the activity measurements, we made sure that the results really correspond to the amount of laccase. These are the most active fractions we have chosen with their contained laccase amount:
- Team Cellulose Binding Domain:
- Colony-PCR of B0034 + GFP_Freiburg; B0034 + GFP_Freiburg + CBDcex; B0034 + GFP_Freiburg + CBDcex_Freiburg; B0034 + GFP_Freiburg + CBDclos_Freiburg.
- All negative
- Restriction of Ecol_Freiburg with XbaI, AgeI and DpnI
- Colony-PCR of B0034 + GFP_Freiburg; B0034 + GFP_Freiburg + CBDcex; B0034 + GFP_Freiburg + CBDcex_Freiburg; B0034 + GFP_Freiburg + CBDclos_Freiburg.
- Team Site Directed Mutagenesis:
Saturday October 20th
- Team Activity Tests:
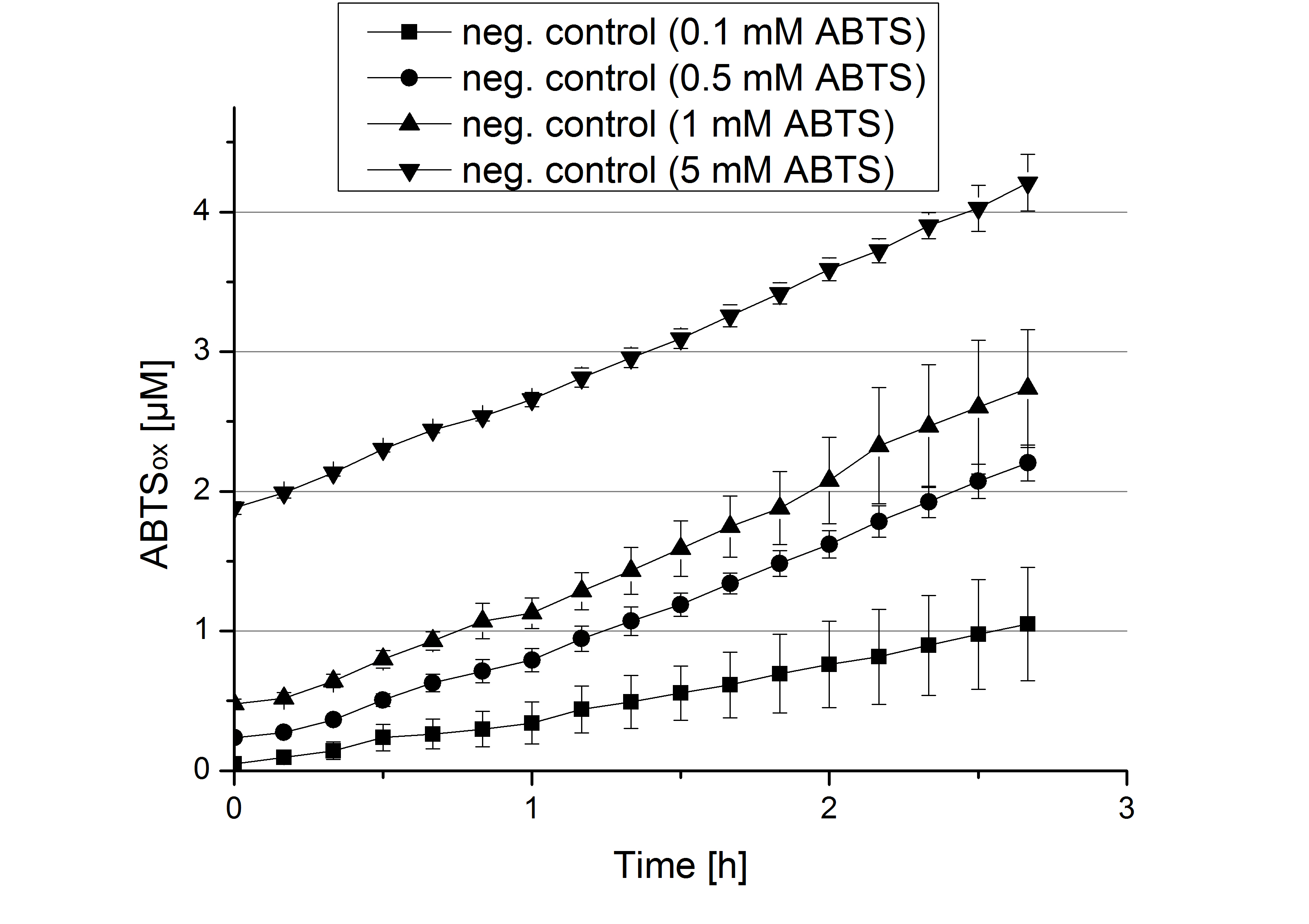
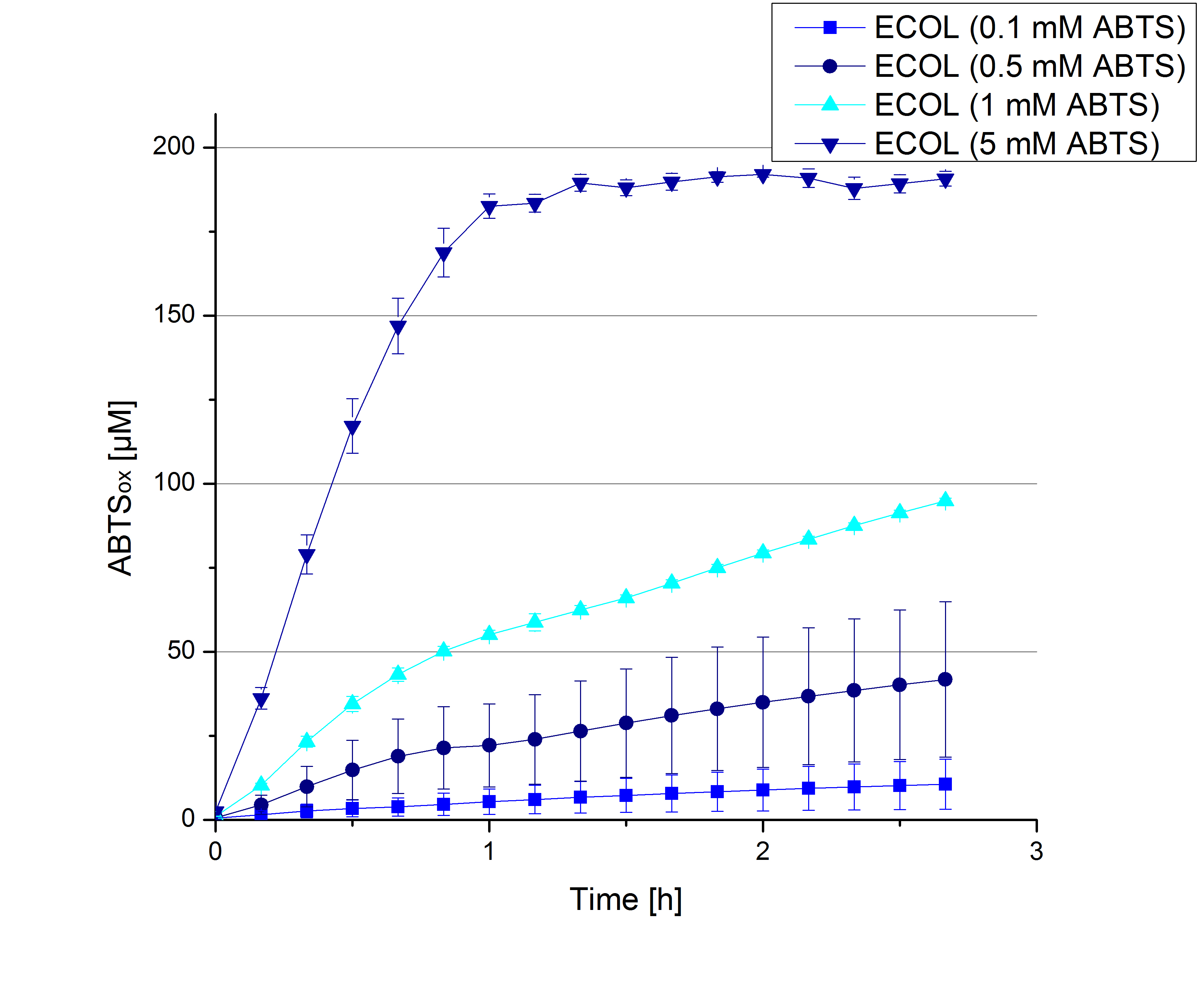
- During the Jamboree in Amsterdam we had the oppurtunity to discuss our activity results with a lot of people. One remark was about the calculation of the enzyme specific units. As you all remember, we measured the activty with 0.1 mM ABTS, but to get comparable results with the literature, we had to measure each protein with other concentrations of ABTS. The concentration of ABTS should be saturated for each laccase. So we started with ECOL and BPUL and applied different ABTS concentrations starting from 0.1 mM as usual: 0.1 mM, 0.5 mM, 1 mM and 5 mM. We applied 616 ng Laccase in each sample. Our results showed, that especially ECOL does not reach its substrate saturation with 5 mM ABTS. Our plans are now to check these concentrations of ABTS for BPUL and TTHL and also higher concentrations of ABTS for all laccases. For that we should reduce the enzyme amount to be able to detect the change in OD 420 properly.
Sunday October 21st
- Team Fungal and Plant Laccases:
Genomic isolation was done with the [http://www.promega.com/resources/protocols/technical-manuals/0/wizard-genomic-dna-purification-kit-protocol/ Promega Wizard genomic DNA purification system kit] of our approximately 50 yeast clones with integrated (a) GFP and (b) tvel5.
- Team Activity Tests:
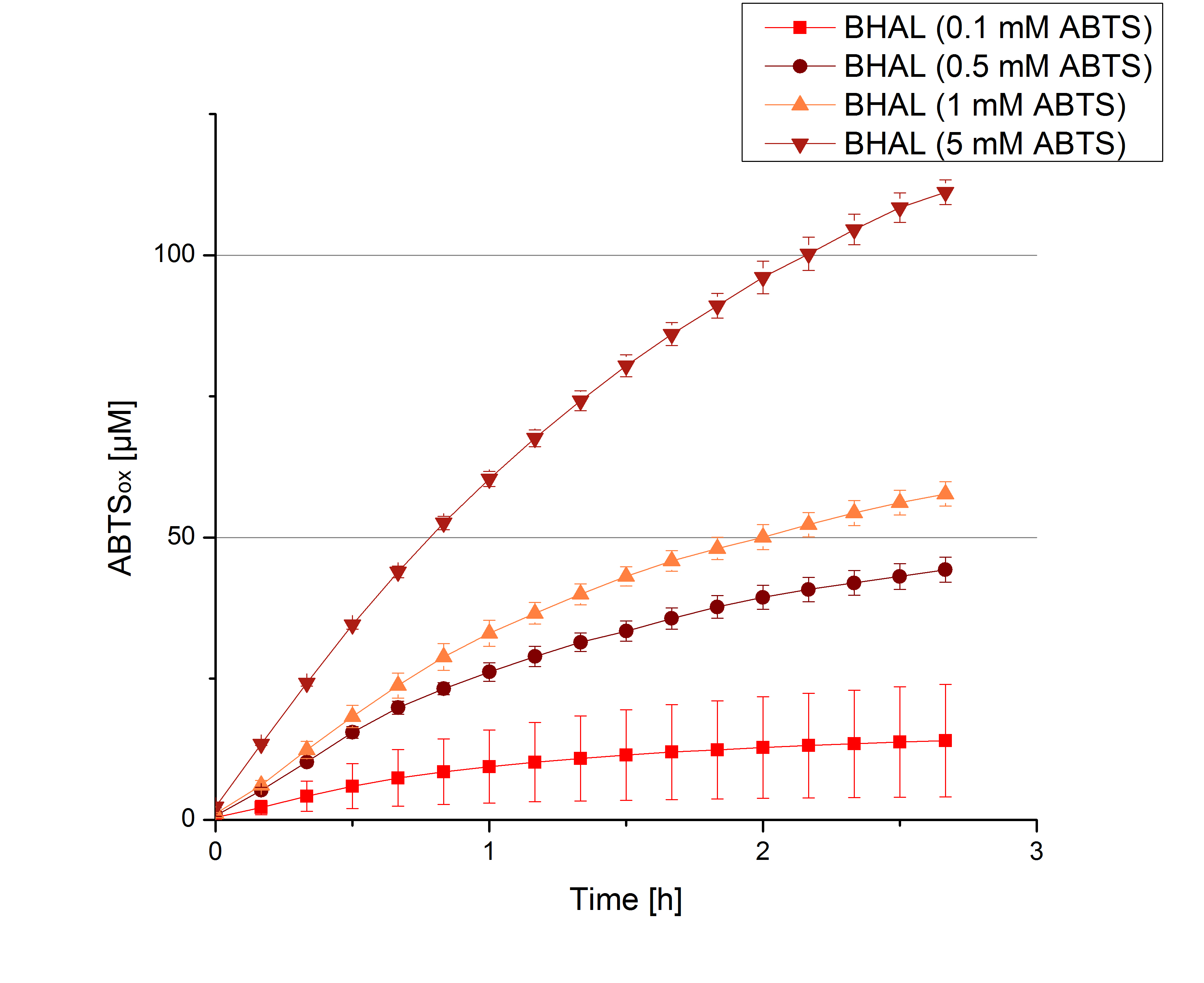
- Today was BHAL's and TTHL's turn. The experiment setup was the same as described the day before. But again we could not make sure, that 5 mM ABTS is the substrate saturation for our tested laccases. We are now sure to go higher in ABTS concentrations.
- Team Cellulose Binding Domain:
- Colonies PCRs of B0034 + GFP_Freiburg; B0034 + GFP_Freiburg + CBDcex_Freiburg; B0034 + GFP_Freiburg + CBDclos_Freiburg
- All negativ
- Transformed once again (with an mix of old an new GFP_Freiburg)
- Isolated plated colonies of transformed colonies (two GFP; two GFP+Cex; two GFP+Clos
- Restriction analysis showed only bands at 2K bp (pSB1C3+BBa_B0034 vector without insert)
- Colonies PCRs of B0034 + GFP_Freiburg; B0034 + GFP_Freiburg + CBDcex_Freiburg; B0034 + GFP_Freiburg + CBDclos_Freiburg
- Team Site Directed Mutagenesis:
- Isolated the plasmids of the six SDM-dishes
- Over-night digestion with NotI
Week 26 (10/22 - 10/28/12)
Contents |
Monday October 22nd
- Team Fungal and Plant Laccases:
The identification of positive yeast clones and determination of the phenotype (M+ or MS) was done by PCR with the primers 5AOX-Phenotype-FW and TT-Phenotype-RV. The clones could be identified and minimal methanol medium was inoculated with them.
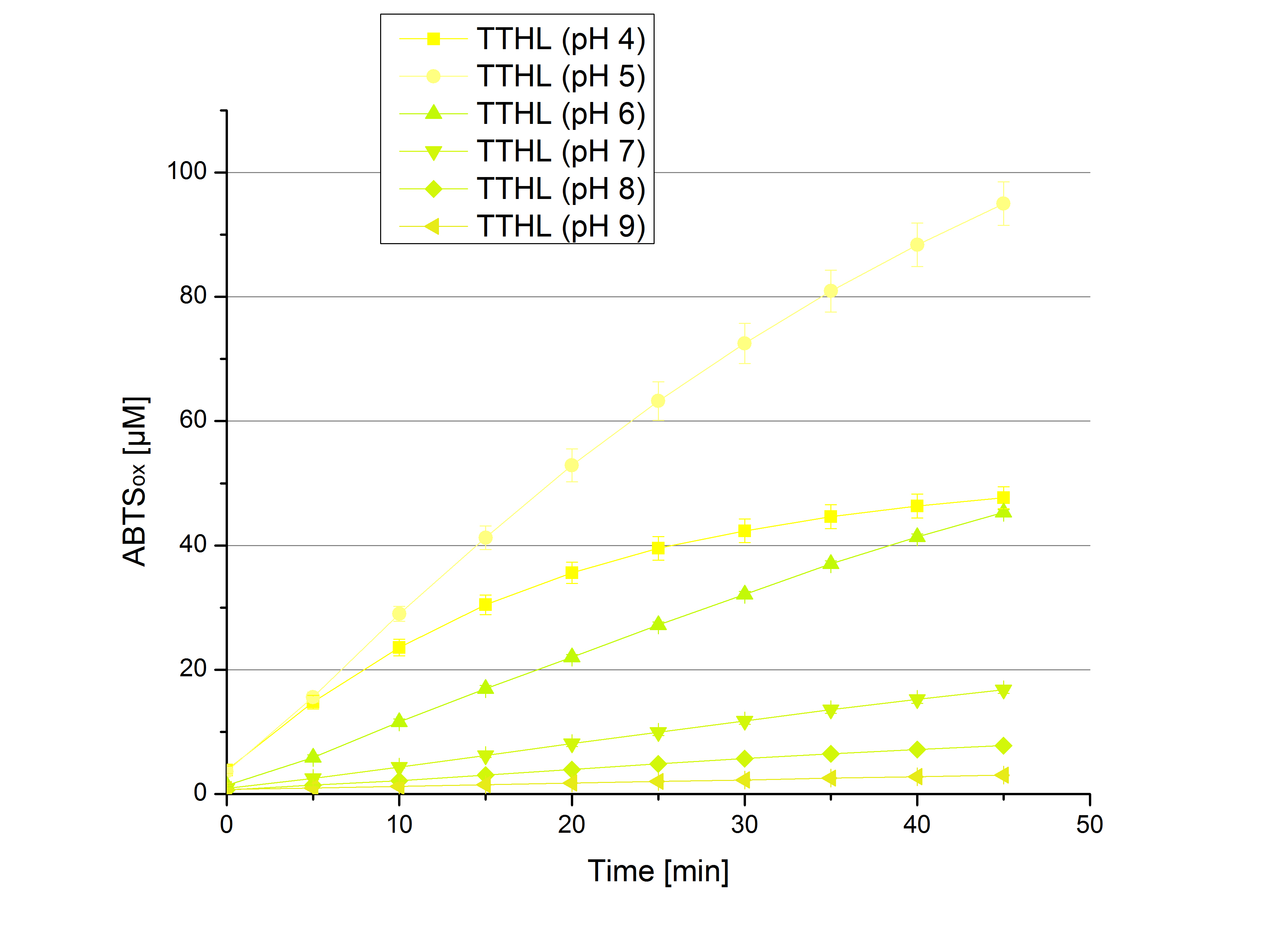
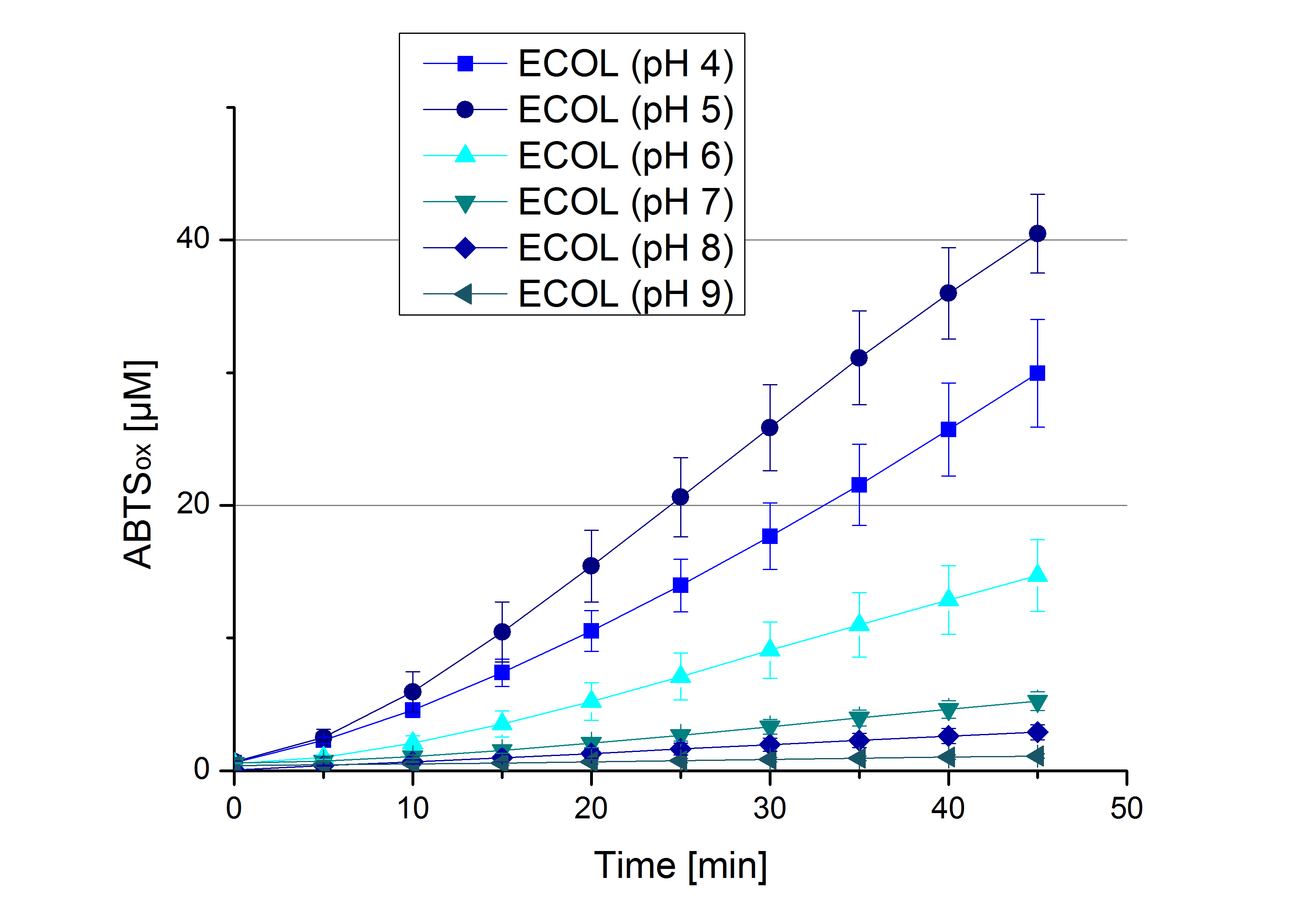
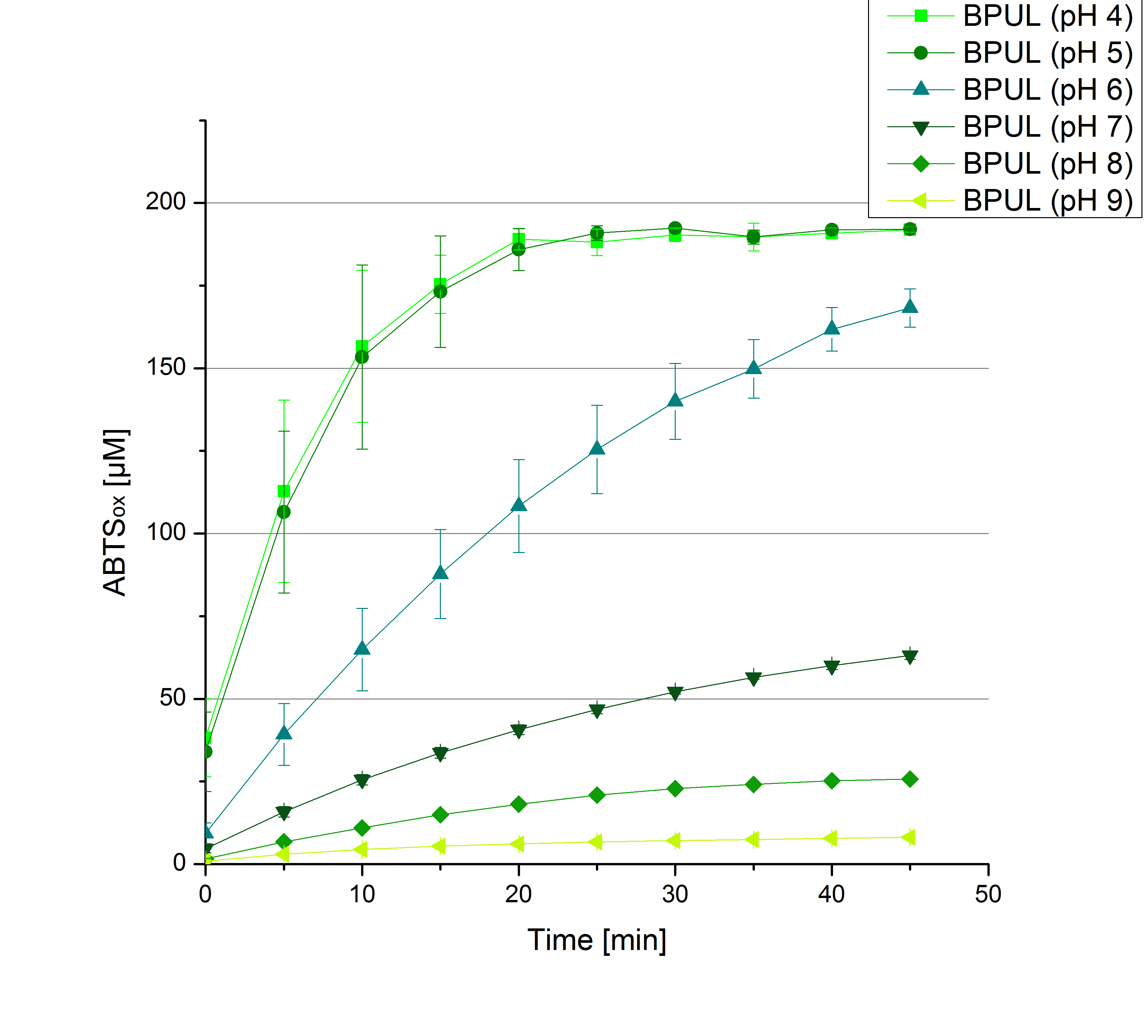
- Team Activity Tests:
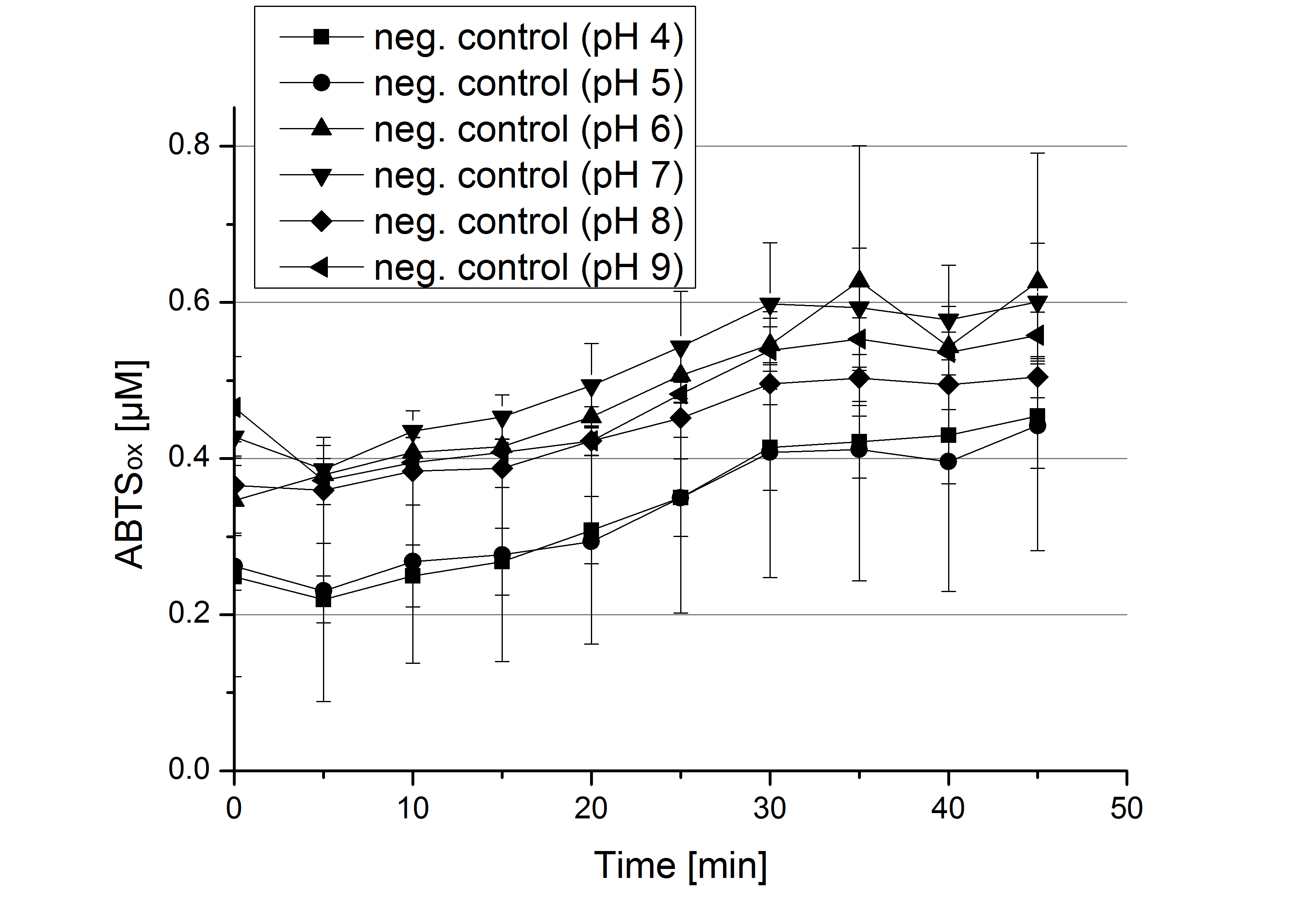
- Today was all about finding a comfy environment for our laccases. We thought of determining the right pH before going on with substrate saturation experiments since we could not be sure, that the activity was influenced by the pH. We chose a range from pH 4 to pH 9, applied 616 ng laccase and 5 mM ABTS. Our results revealed the following results:
- optimal pH of ECOL: pH 5
- optimal pH of BPUL: pH 4 and pH 5
- optimal pH of BHAL: pH 5
- optimal pH of TTHL: pH 5
-
- Team Cellulose Binding Domain:
- Sequencing of <partinfo>BBa_K863120</partinfo> in <partinfo>BBa_J61101</partinfo> showed a deletion within the ORF of the GFP; this seemed to be the reason that the colonies are not fluorescent.
- Gradient-PCR on <partinfo>BBa_I13522</partinfo> with GFP_Freiburg Pre- and Suffix Primers
- Worked fine. Clean up and digestion with XbaI and PstI
- Gradient-PCR on <partinfo>BBa_K863103</partinfo> with CBDcex_Freiburg and GFP_Freiburg_compl Primers
- One of 12 temperatures worked. Clean up and digestion with XbaI and PstI
- Gradient-PCR on <partinfo>BBa_K863113</partinfo> with CBDclos_Freiburg and GFP_Freiburg_compl Primers
- did not get product at all
- Ligation of the PCR products and GFP_Freiburg and CBDcex_Freiburg-GFP_Freiburg, respectively
- Transformation of both ligations
- Cells plated on selection agar
- Team Site Directed Mutagenesis:
- Stopped Digestion of the shuttle vector, but gel went wrong and the products were lost
Tuesday October 23rd
- Team Fungal and Plant Laccases: The induction of the yeast cells with 1% (v/v) 100% methanol was done at morning and in the evening.
- Team Activity Tests:
- In order to find the substrate saturation we continued to measure our laccase activities with higher concentrations of ABTS. To have a slower reaction and also try not to waste the enzymes we halfed the amount of used laccases from 616 ng to 308 ng of each laccase.
- Team Cellulose Binding Domain:
- Colony PCR of B0034 + GFP_Freiburg
- 14/24 positive
- Plated three on selection agar for plasmid isolation
- Colony PCR of B0034 + CBDcex-GFP_Freiburg
- 16/24 positive
- Plated three on selection agar for plasmid isolation
- Colony PCR of B0034 + GFP_Freiburg
Wednesday October 24th
- Team Fungal and Plant Laccases: In the morning and evening the induction of yeast cells with 1% (v/v) methanol was done.
- Team Cellulose Binding Domain:
- Isolated Plasmids containing B0034 + GFP_Freiburg &
- Digestion of pooled plasmids with XbaI and PstI
- Isolated Plasmids containing B0034 + CBDcex-GFP_Freiburg
- Digestion of pooled plasmids with XbaI and PstI
- Digestion of J23100 with SpeI and PstI
- Clean up of inserts (B0034 + GFP_Freiburg & B0034 + CBDcex-GFP_Freiburg) and Backbone (J23100) via gel
- Ligation of Backbone and the inserts
- Transformation and plated on selection agar
- Isolated Plasmids containing B0034 + GFP_Freiburg &
- Team Site Directed Mutagenesis:
- Digestion showed that all isolated colonies still have the illegal XbaI restriction side
Thursday October 25th
- Team Fungal and Plant Laccases:
- Induction of the yeast cells with 1% (v/v) methanol was done. After 4 h the cells were harvested by centrifugation at 3000xg 4°C for 5 min. After adjusting buffer composition of the supernatant the activity test was done.
- Team Cellulose Binding Domain:
- All colonies did not show any obvious green glow
- Team Substrate Anaylsis:
- The MS-MS data arrived from Marcus Persicke. To ensure our measurements and discuss what happens after the Laccase treatment and on the ESI we decided to ask an organic chemistrer so we went to Prof. Dr. Dietmar Kuck whos expert on this field. Since he had less time to discuss we have only speculativ testifies.
Friday October 26th
- Wiki day
Saturday October 27th
Sunday October 28th
| 55px | | | | | | | | | | |
 "
"