Team:NTU-Taida/Result/Secretion-GLP1
From 2012.igem.org
Darlisereno (Talk | contribs) |
Darlisereno (Talk | contribs) |
||
| Line 4: | Line 4: | ||
==Method== | ==Method== | ||
| - | To check whether our bacteria successfully | + | To check whether our bacteria successfully secrete GLP-1 to the environment, we collected the bacterial media and measure its GLP-1 concentration. After centrifugation of bacterial culture, we collected the supernatant and passed it through 0.22 μm filter to exclude bacteria. Since the secreted GLP-1 is too diluted by the large volume of media, we concentrated the solution by 5 kDa ultra centrifugal filter tubes. We also collected the bacterial pellet and add lyze it by ''E. coli'' lysis buffer and freeze-thaw to measure GLP-1 inside bacteria. Both Immunoblotting and ELISA are used to detect GLP-1 concentration inside and outside bacteria. |
==Protocol== | ==Protocol== | ||
| - | |||
===A. Sample Preparation=== | ===A. Sample Preparation=== | ||
| - | # | + | #310 μL Bacterial culture was cultured in 5mL LB at 37℃, with suitable concentration of antibiotics shaking till 600 nm absorbance in cuvette is 1.5 (usually it takes about 18 hr). |
#Centrifuge the bacterial culture at 12000 rpm for 3 min. | #Centrifuge the bacterial culture at 12000 rpm for 3 min. | ||
#Pass the supernatant through 0.22 μm filter. Transfer the supernatant to 5 kDa ultra centrifugal filter tubes and centrifuge at 10000 rpm for 40 min. | #Pass the supernatant through 0.22 μm filter. Transfer the supernatant to 5 kDa ultra centrifugal filter tubes and centrifuge at 10000 rpm for 40 min. | ||
| - | #Add | + | #Add 250 μL of ''E. coli'' lysis buffer to the pellet and use liquid Nitrogen to freeze-thaw for three cycles. |
| - | #Heat the samples at | + | #Heat the samples at 95。C for 10 min before immune-blotting. |
===B. Dot-Blotting=== | ===B. Dot-Blotting=== | ||
| - | #Soak the PVDF membrane in ethanol for | + | #Soak the PVDF membrane in ethanol for 5 min to activate the membrane. |
#Drop every 2 μL sample in a very small area on the membrane. | #Drop every 2 μL sample in a very small area on the membrane. | ||
| - | #Blocking with 5% de-fat milk (in PBST) for | + | #Blocking with 5% de-fat milk (in PBST) for 1 hr. |
#Wash with PBST for 10 min, repeat 3 times. | #Wash with PBST for 10 min, repeat 3 times. | ||
| - | #Add primary antibody (1:1000 dilution in PBST) and incubate at | + | #Add primary antibody (1:1000 dilution in PBST) and incubate at 4℃ overnight. |
#Wash with PBST for 10 min, repeat 3 times. | #Wash with PBST for 10 min, repeat 3 times. | ||
| - | #Add secondary antibody (1:5000 dilution in PBST) and incubate at 25℃ | + | #Add secondary antibody (1:5000 dilution in PBST) and incubate at 25℃ 2 hr. |
#Wash with PBST for 10 min, repeat 3 times. | #Wash with PBST for 10 min, repeat 3 times. | ||
#Mix 2 mL ECL with 2mL substrate, add to the membrane and let stand for 5 min. | #Mix 2 mL ECL with 2mL substrate, add to the membrane and let stand for 5 min. | ||
#Expose the membrane to the film in dark room. | #Expose the membrane to the film in dark room. | ||
| + | |||
===C. ELISA=== | ===C. ELISA=== | ||
| Line 33: | Line 33: | ||
#Decant liquid from plate. | #Decant liquid from plate. | ||
#Wash 5 times with 250 μL Wash Buffer. | #Wash 5 times with 250 μL Wash Buffer. | ||
| - | #Add 200 μL Detection Conjugate in each well. Incubate | + | #Add 200 μL Detection Conjugate in each well. Incubate 2 hr at room temperature. |
#Wash 3 times with 250 μL Wash Buffer. | #Wash 3 times with 250 μL Wash Buffer. | ||
#Add 200 μL diluted substrate and incubate for 20 min. | #Add 200 μL diluted substrate and incubate for 20 min. | ||
#Read plate on fluorescence plate reader with excitation/emission wavelength of 355 nm/460 nm. | #Read plate on fluorescence plate reader with excitation/emission wavelength of 355 nm/460 nm. | ||
| + | |||
==Data== | ==Data== | ||
Revision as of 22:21, 26 September 2012
Secretion: GLP-1
Contents |
Method
To check whether our bacteria successfully secrete GLP-1 to the environment, we collected the bacterial media and measure its GLP-1 concentration. After centrifugation of bacterial culture, we collected the supernatant and passed it through 0.22 μm filter to exclude bacteria. Since the secreted GLP-1 is too diluted by the large volume of media, we concentrated the solution by 5 kDa ultra centrifugal filter tubes. We also collected the bacterial pellet and add lyze it by E. coli lysis buffer and freeze-thaw to measure GLP-1 inside bacteria. Both Immunoblotting and ELISA are used to detect GLP-1 concentration inside and outside bacteria.
Protocol
A. Sample Preparation
- 310 μL Bacterial culture was cultured in 5mL LB at 37℃, with suitable concentration of antibiotics shaking till 600 nm absorbance in cuvette is 1.5 (usually it takes about 18 hr).
- Centrifuge the bacterial culture at 12000 rpm for 3 min.
- Pass the supernatant through 0.22 μm filter. Transfer the supernatant to 5 kDa ultra centrifugal filter tubes and centrifuge at 10000 rpm for 40 min.
- Add 250 μL of E. coli lysis buffer to the pellet and use liquid Nitrogen to freeze-thaw for three cycles.
- Heat the samples at 95。C for 10 min before immune-blotting.
B. Dot-Blotting
- Soak the PVDF membrane in ethanol for 5 min to activate the membrane.
- Drop every 2 μL sample in a very small area on the membrane.
- Blocking with 5% de-fat milk (in PBST) for 1 hr.
- Wash with PBST for 10 min, repeat 3 times.
- Add primary antibody (1:1000 dilution in PBST) and incubate at 4℃ overnight.
- Wash with PBST for 10 min, repeat 3 times.
- Add secondary antibody (1:5000 dilution in PBST) and incubate at 25℃ 2 hr.
- Wash with PBST for 10 min, repeat 3 times.
- Mix 2 mL ECL with 2mL substrate, add to the membrane and let stand for 5 min.
- Expose the membrane to the film in dark room.
C. ELISA
- Wash the wells with 250 μL Wash Buffer.
- Add 100 μL Assay Buffer to each well, and add 100 μL standards in ascending orders to wells, and add samples in the remaining wells.
- Incubate at 4℃ overnight.
- Decant liquid from plate.
- Wash 5 times with 250 μL Wash Buffer.
- Add 200 μL Detection Conjugate in each well. Incubate 2 hr at room temperature.
- Wash 3 times with 250 μL Wash Buffer.
- Add 200 μL diluted substrate and incubate for 20 min.
- Read plate on fluorescence plate reader with excitation/emission wavelength of 355 nm/460 nm.
Data
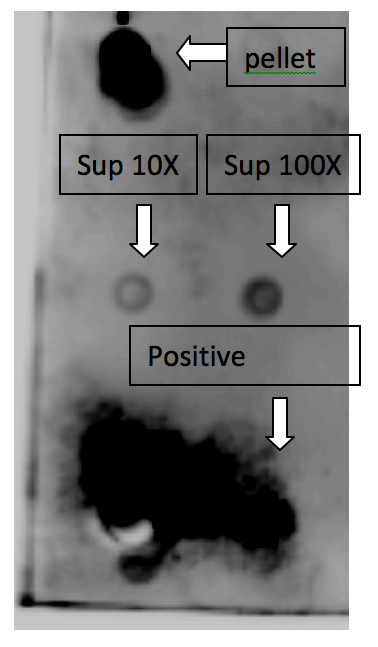
A. Dot-Blotting
The upper dot is from the lyzed bacterial pellet, which represents the GLP-1 inside the bacteria. The middle part is from bacterial culture supernatant, which means the secreted GLP-1, and the left dot is the media we did 10X concentration, the right dot is the one we did 100X concentration. The lower dot is from pure GLP-1 solution as positive control.
B. ELISA
The left bar is from the lyzed bacterial pellet, which represents the GLP-1 inside the bacteria. The right bar is from 10X concentrated bacterial culture supernatant, which means the secreted GLP-1.
Conclusion
Our data indicates that although there is much GLP-1 inside the bacteria, some of GLP-1 is secreted by our signal peptide design. The secreted GLP-1 can be delivered to intestinal lumen and carry out its physiological function once absorbed.
 "
"