Team:NTU-Taida/PEPDEX
From 2012.igem.org
(→stabilization modules) |
(→modeling and application) |
||
| Line 152: | Line 152: | ||
==== '''modeling and application''' ==== | ==== '''modeling and application''' ==== | ||
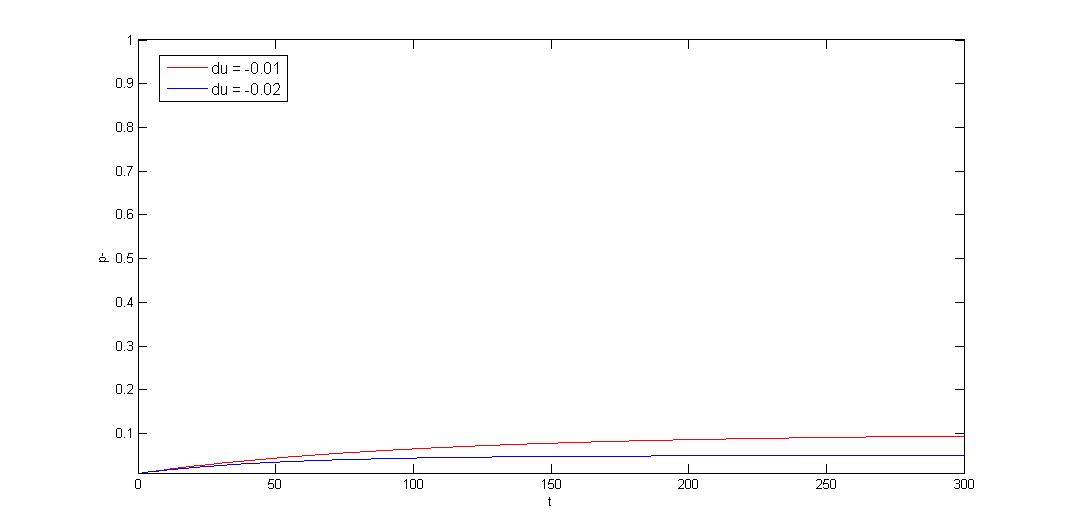
| - | + | <h3>How to model plasmid instability:</h3> | |
| + | [[FIle:NTU-Taida-Negative du.jpg|400px|center]] | ||
=== '''safety of delivery system''' === | === '''safety of delivery system''' === | ||
Revision as of 11:16, 22 September 2012
Project
Testing Hero
Contents |
Overall project
In our project, we aim to utilize a microbe that responds to conditions in human body as an approach to administer smart peptide-based therapies. GLP-1, a human innate neuro-peptide for energy balance, is chosen to combat for obesity and metabolic syndrome. We engineer the non-pathogenic E. coli which senses fatty acids in intestines and secretes synthetic GLP-1. Appropriate signal peptides and penetratin are used to facilitate peptide secretion and intestinal uptake. Furthermore, we design a circuit with quorum sensing and double repressors, which aims to generate quick but sustainable responses and serves as an anti-noise filter. Plasmid stabilization modules including partition system and multimer resolution system are also incorporated to circumvent the undesirable loss or segregational instability of our artificial device. With this general concept of delivery of short peptide into human body, we can also target other human diseases with alternative circuit designs.
Project Details
Enteral delivery of neuro-peptide for weight and metabolic control
Design
CPP File:Https://www.dropbox.com/s/1lnt1d2uo401rz6/123.mov fadR
Modeling
Results
Extension
peptide2
Background
Design
Modeling
Results
Extension
Thermal promoter
Phs thermal promoter
E. coli is pretty sensitive and responsive to temperature changes. We brought promoter Phs, located within dnaG, and transient induced to temperature upshift, to our circuit. As described by Wayne E. Talyor et al., Phs can forward upregulation of a series of proteins. The increase activity of Phs promoter is partially compatible with the increase synthesis of sigma factor. The quick respsonse of Phs is suitable since its the sigma factor synthesis peaks 10 minutes after the temperature upshift, and so does Phs RNA levels. The increase ratio before and after the temperature can be more than 20 folds. On particular note, Phs is lack of consensus region over -10 region, which explains its poor activity in 30 Celsuius degree. Thus, Phs is marked as a candidate in our circuit, which can only be functional after ingestion into human body.
Modified promoter CI with CIts
This circuit involves a thermosensitive CI promoter under the expression of a strong promoter, J23119, and is followed by a pCI promoter region and reporter of desire. The whole composite circuit is designed by Harvard iGEM team, 2008. Under conditions demonstrated before, its increase of reporter expression after temperature upshift is minimal (1.8 fold). And thus, we design novel pCI promoter region with alterer binding affinity to CIts repressor in search of better efficiency.
Secretory System
Gram-negative bacteria have several ways to secrete their protein products, and these secretory systems had been categorized into six types, from type I secretory system(T1SS) to type VI secretory system(T6SS). Every type of system has its own unique character. Due to the product we planned to produce, GLP-1 and CPP(penetratin), we decided to use Type II secretory system (T2SS) for several reasons as following. First, T2SS are believed to be normally expressed in Escherichia coli(E. coli.). Second, some T2SS(Sec pathway) are believed to be self-degrading for their signal peptides. Third, some T2SS(Tat pathway) are believed to be capable of secreting folded protein. In order to secrete protein by T2SS, you need to first decide which signal peptide to attach to your protein. Then, the signal peptie will be recognized by either Sec, SRP or Tat pathway to be transported to periplasm and throught a common transporter protein into the extracellular environment.(figure 1)
After deciding which type of secretory system we would use, we started to look out for our signal peptide candidate. We chose our three candicates based on several review papers (J. H. Choi et al, 2004) and we found out the three original papers(2,3,4). Due to the limitation of time and money, we de novo construct our signal peptides(SP) to be linked to our products(GLP-1 and CPP).(figure 2) We then constructed them with constitutively-expressed promoter and RBS. With this construct done, in both DH5α and BL-21 strain, our E. coli. can be used to produce our target protein-signal peptide complex, also they can be used to test if the signal peptide and secretory system really work. To sense those proteins the E. coli. secreted, we used Dot-blotting and ELISA tests. The results are devided into two parts, Dot-blotting and ELISA.
Dot-blotting
Dot-blotting is a technique for detecting target protein within a cell. We remodified the standard dot blotting protocol to meet our needs. Our aim is to sense the protein the E. coli. secreted, so instead of collecting and deviding the insoluble and soluble target protein within the bacteria, we sensed the protein and compared the protein amount extracellularly and intracellularly.
Our first dot-blotting is for both practicing and condition optimization, so we strictly followed standard protocol for comparing intracellular protin content.(figure)
ELISA
Stability and safety
Every GM system that will function outside lab will face two major problems:system stability and safety! When the GM bacteria live
Stability of delivery system
Briefing
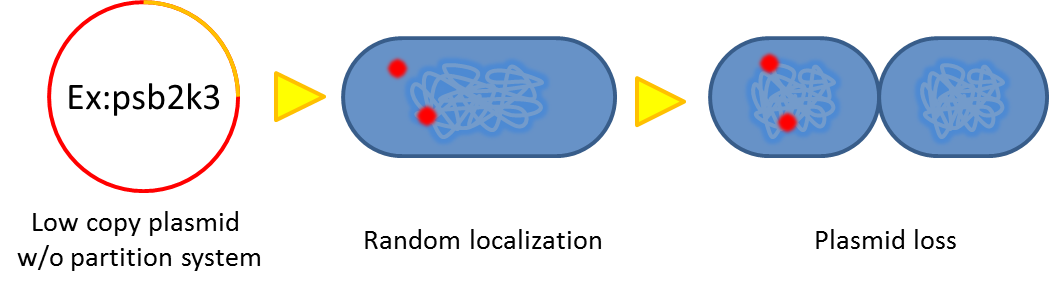
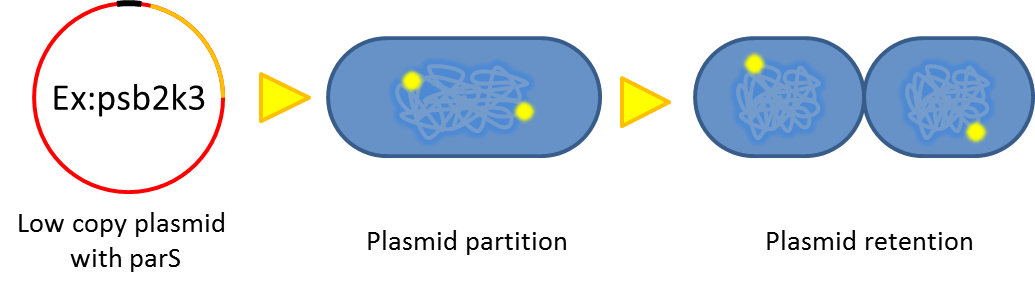
As our system will function outside the labrotory and human gut lack for antibiotic selection pressure, the vector stability is the critical point that determine whether our system is applicable or not. Inspired by natural plasmid & mobile gene element, we cope up with vector instability by incorporating partition system, Multimer resolution system and toxin antitoxin system these modules into our design.
Background
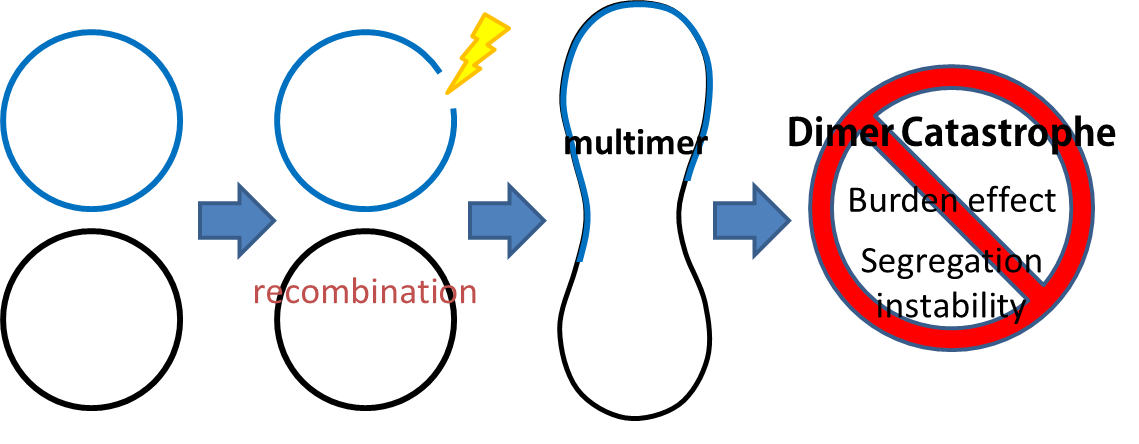
Sources of plasmid instability:
- segregational instability
- burden effect
- dimer catastrophe
- A description list is perfect for defining terms.
stabilization modules
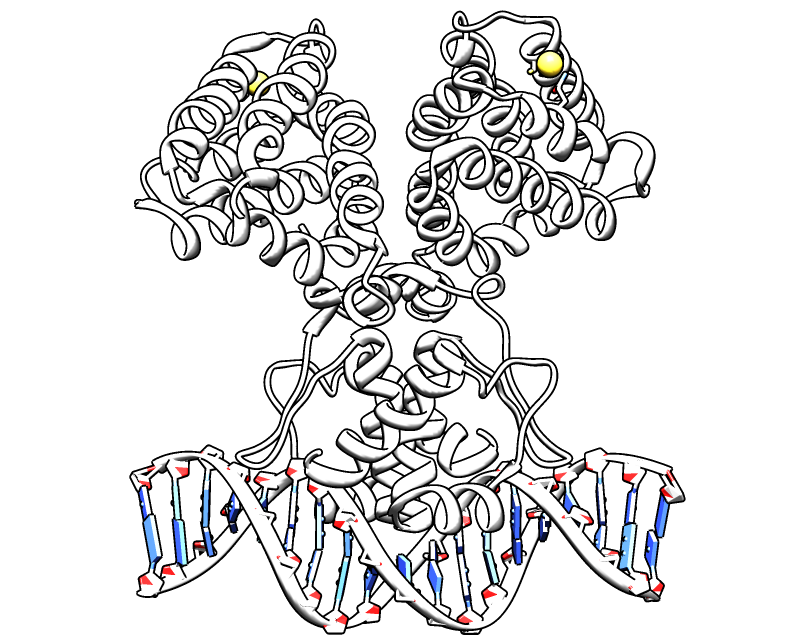
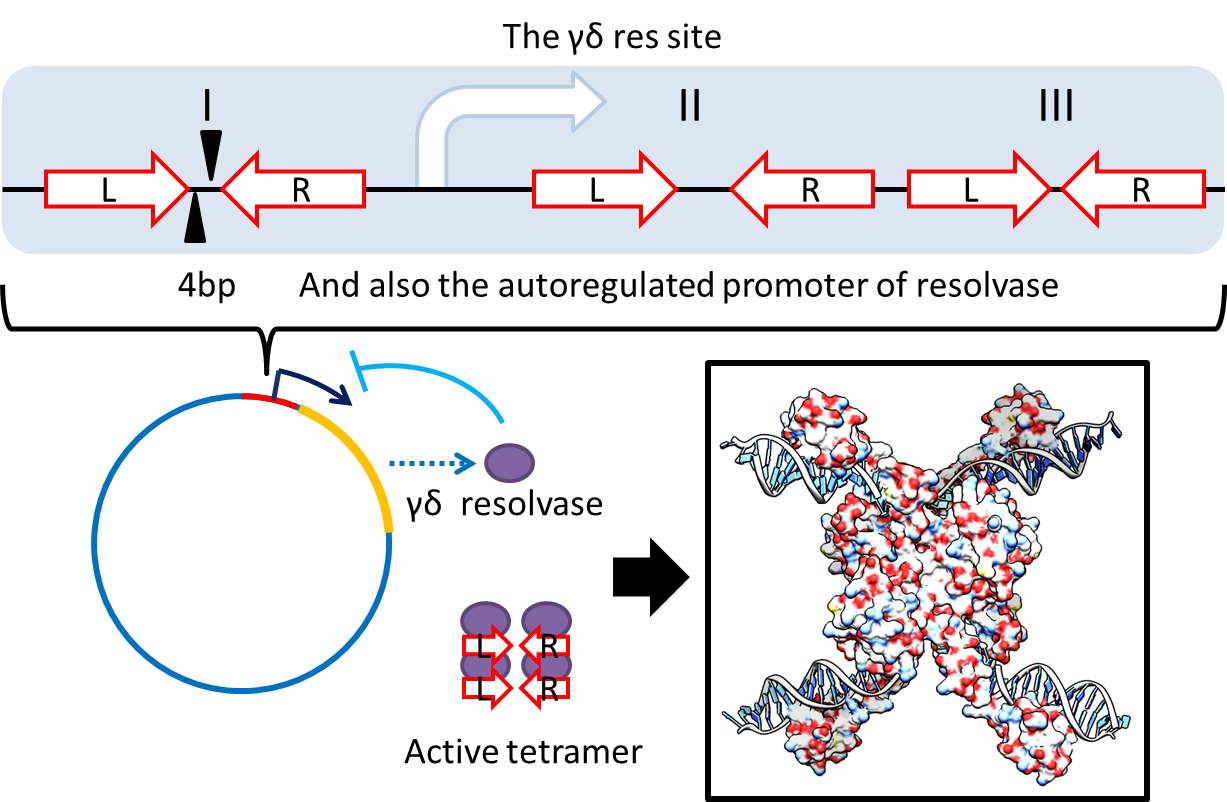
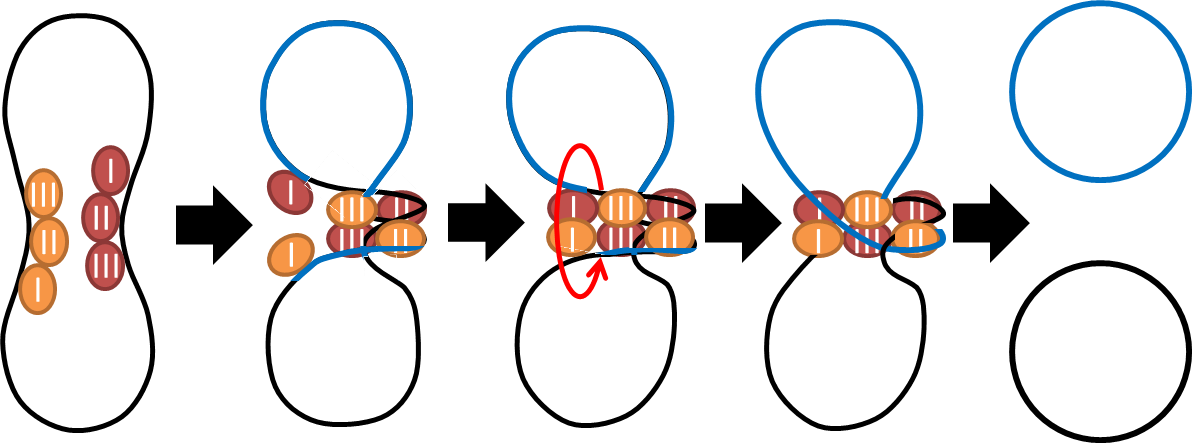
multimer resolution system:Tn1000(gamma delta) resolution system
This cassette encodes an autoregulated resolvase(serine type recombinase)from E.coli F plasmid transposon tn1000(tn3 family). Its promoter region consists of 3 sub-sites(res site) and can process recombination. This cassette can resolve multimer that formed during replicative transposition, so it can also resolve plasmid multimer providing plasmid stability by avoiding dimer catastrophe. For this reason, it is multimer resolution system(MRS) that provide the same function as E.coli chromosome XerCD/dif but acts independent of cell cycle and may have higher efficiency on plasmids compared with slow XerCD system.
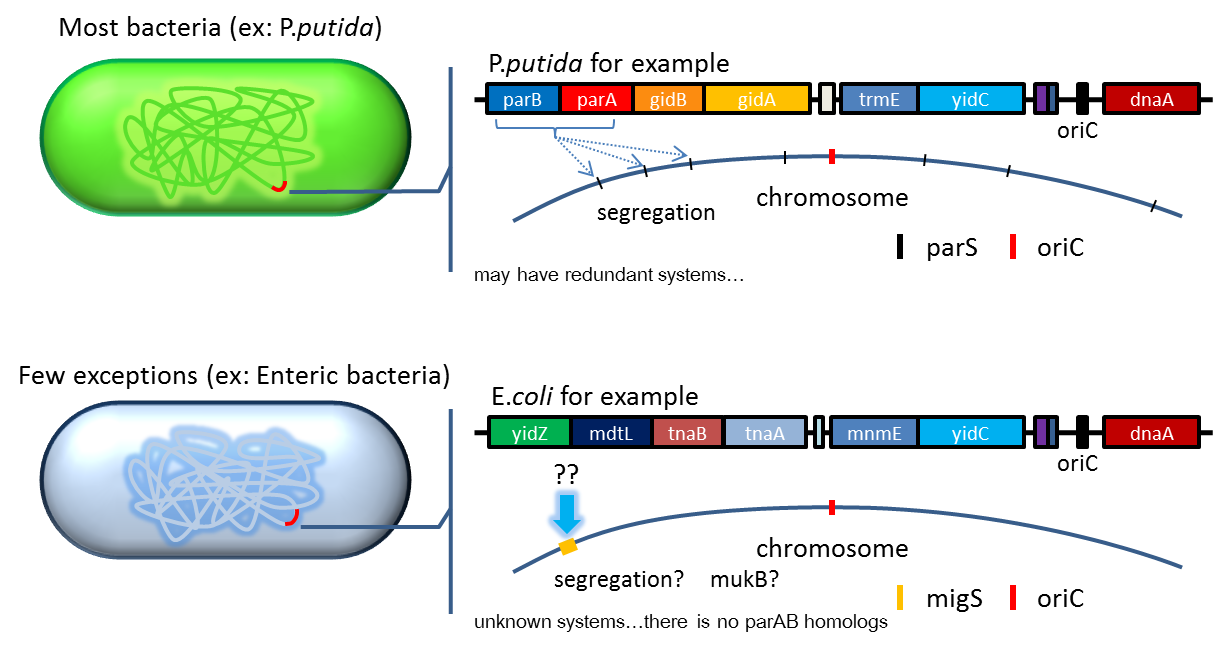
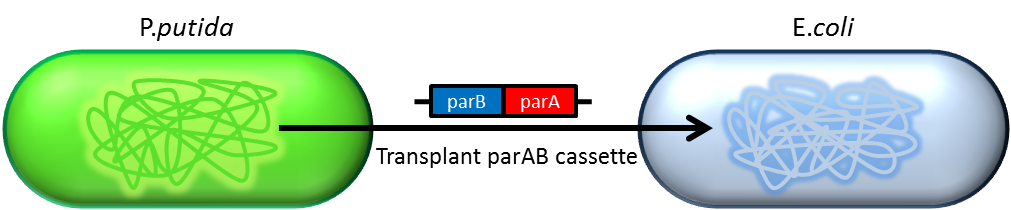
partition system:from pseudomonas putida KT2440
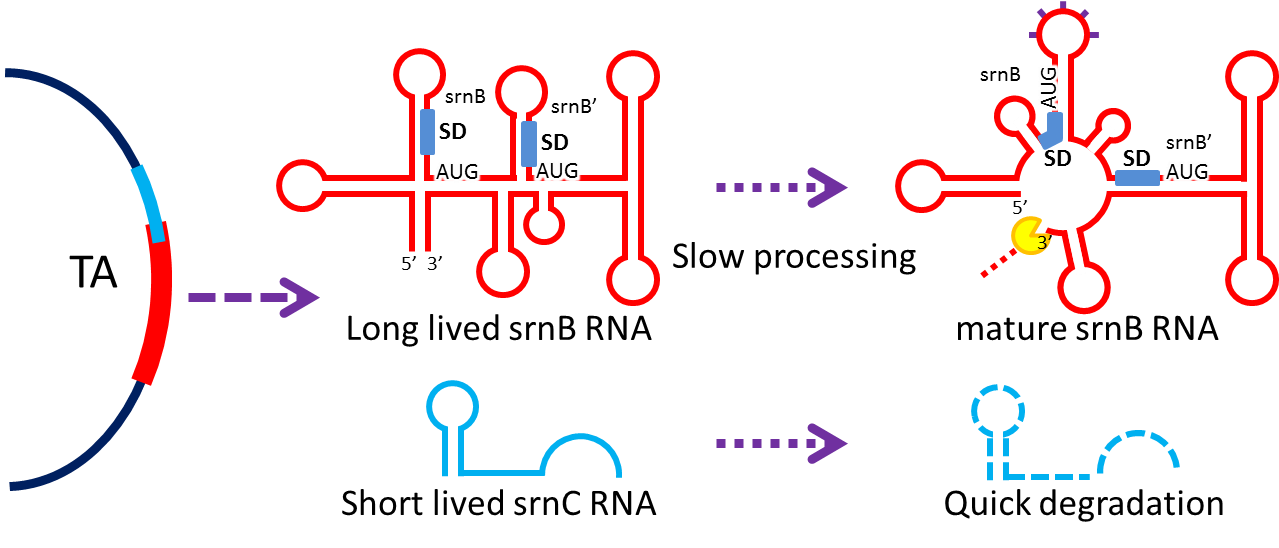
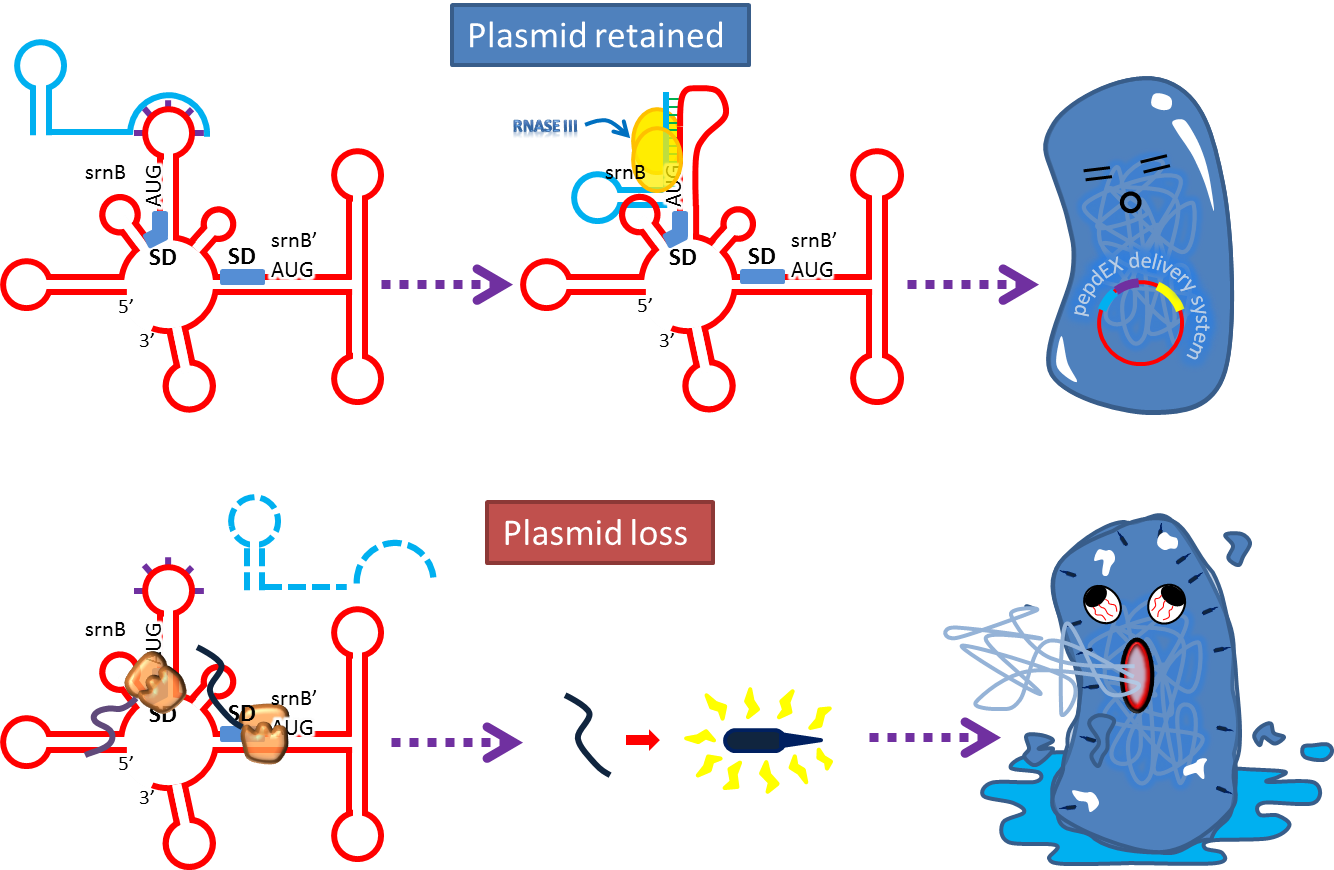
Post-segregation killing:srnBC toxin-antitoxin system
It is type I toxin-antitoxin which belongs to hok/sok homologues, it expresses stable toxin encoded RNA and short lived antitoxin RNA that can neutralize toxin RNA by RNA interaction. It acts as post segregation killing system, which kills bacteria when it loss the DNA(genomic islands, plasmids, mobile gene elements) that contains it. Therefore we use it to reduce plasmid loss rate and make applications without antibiotics selection pressure more feasible.
Besides these modules, reducing the burden effect is also important to the system stability. Well designed system and lower gene dosage may help.
| HEAD | head |
|---|---|
| 1 | 2 |
| 3 | 4 |
| 5 | 6 |
reference
- 1.reference one
- 2.reference two
modeling and application
How to model plasmid instability:
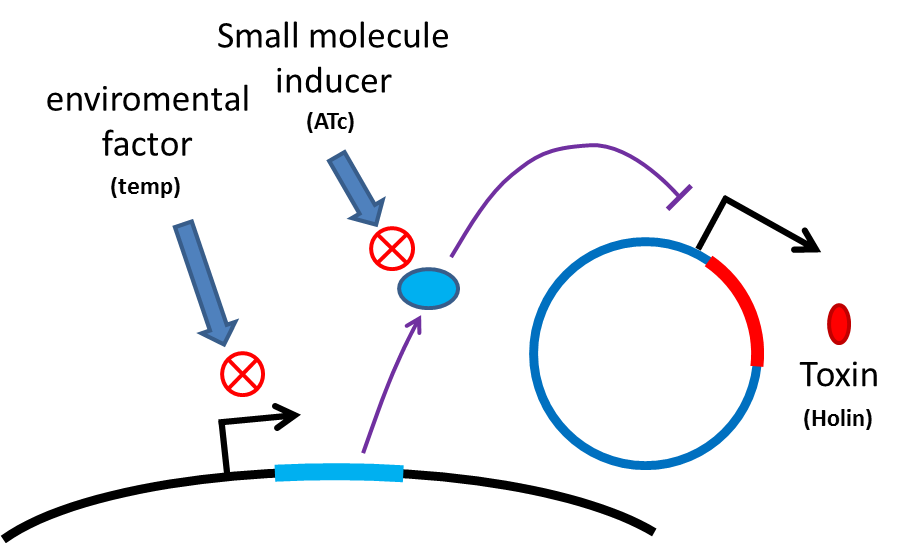
safety of delivery system
Many turn off strategies have been developed, most of these are the inducible suicide system that can be activated at certain condition. For instance, in our project, we plan to use temperature and small molecule as activating signals( following picture). When the course of treatment ends administration of small amount of tetracycline agonist will induce bacterial death, leaving human body also cause suicide gene activation thereby avoid recombinant strain/gene pollutiing. And splitting suicide system to provide repression in trans can prevent plasmid transfering to wild type strains.
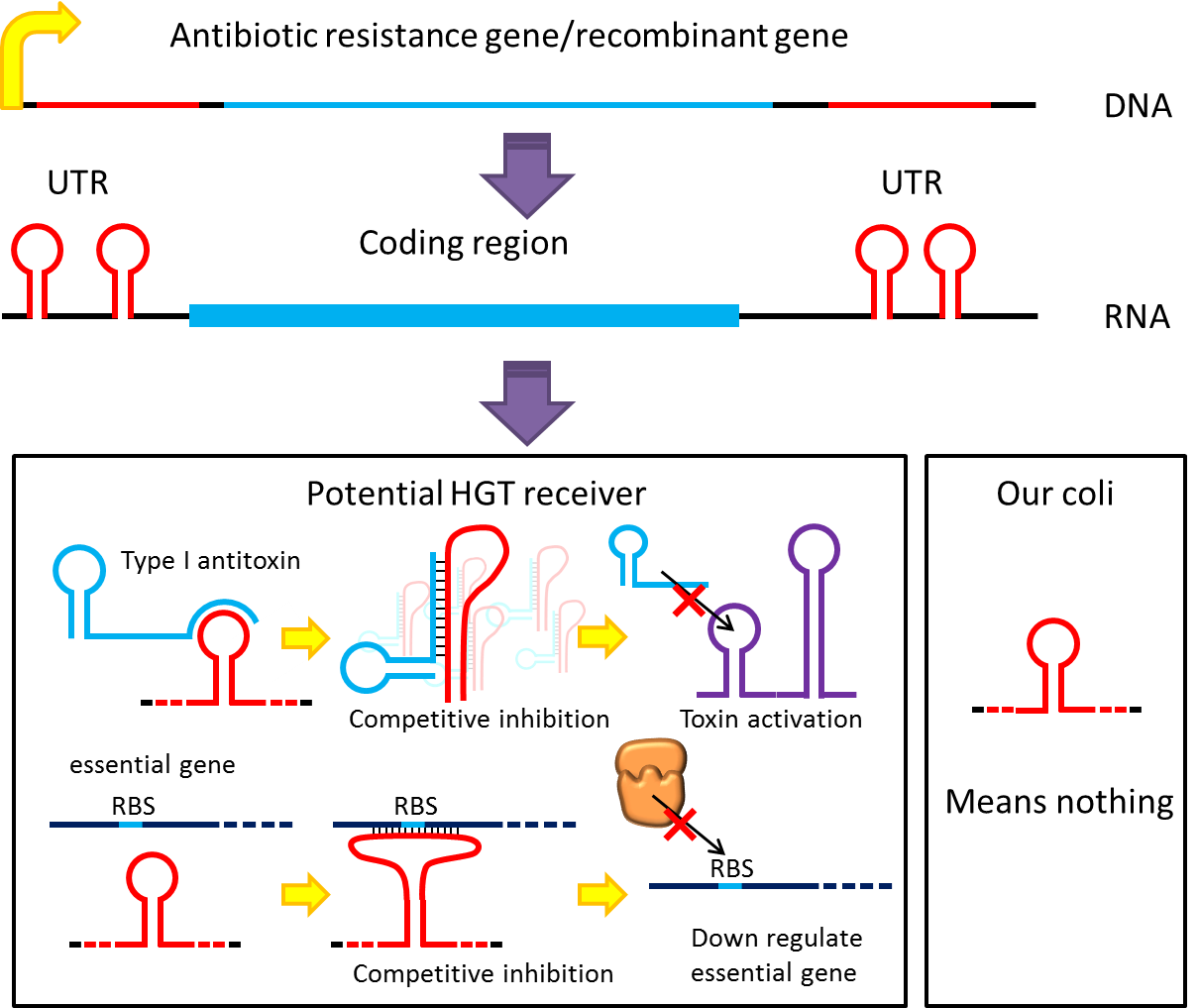
However, these design cannot totally eliminate the risk of horizontal gene transfer(HGT), which recombinant genes can move to other organisms independent of suicide system. So besides suicide system, we have a new idea to deal with these kinds of HGT risks by RNA interaction.
Expression well designed antisense RNAs have been shown to have inhibitory effect on target RNAs, and peptide nucleic acid (PNA) that antisense to antitoxin 5’RNA can cause bacteria death. Putting appropriate antisense RNAs on untranslated region of transcripts may interfere target RNA function or translation. This property can be used to prevent HGT. For instance, HGT is more likely to occur between related species, laboratory E.coli have inactivated all its hok/sok toxin-antitoxin system by mutation, but wild bacteria especially pathogenic bacteria have many active TA locus on its chromosome like E.coli O157 have many active hok/sok homologs. we plan to put a stem loop from hok mRNA which can pair with sok RNA 5’sequence on UTR of antibiotic resistance genes we used. IF wild E.coli steal our antibiotic resistance genes and express it, its antitoxin will be competitive inhibited and its toxin will express and kill the thief thus preventing HGT between lab & wild coli. This idea can have wide extension. Besides targeting antitoxin (functional RNA) of type I TA, designing antisense sequences that target RBS to down regulate targeted protein is also possible. Targeting antitoxin of type II TA, essential genes for metabolism, housekeeping genes and any sequences exist in potential HGT receiver but not our coli can be used. Even if the design cannot kill thieves, it can weaken receivers and reduce advantages antibiotic genes bring about thus reduce possibility and danger of HGT.
In the past the repression efficiencies of antisense RNA in bacteria are low, but by using the paired termini antisense RNA method or incorporate U turn/YUNR motif, these applications will become more and more feasible.
Level 1 of column
Modeling
Results
| HEAD | head |
|---|---|
| 1 | 2 |
| 3 | 4 |
| 5 | 6 |
Extension
 "
"