Team:HokkaidoU Japan/Notebook/plastic Week 9
From 2012.igem.org
| (71 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
<!-- DO NOT EDIT OVER THIS LINE @iTakeshi --> | <!-- DO NOT EDIT OVER THIS LINE @iTakeshi --> | ||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | == | + | ===August 27th=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | + | [[image:HokkaidoU2012 120827 phaC digestion sugama.jpg|thumb|digestion result]] | |
| - | ==Digestion== | + | [[image:HokaidoU2012 120827 RBS digestion sugama.jpg|thumb|digestion result]] |
| - | + | ====Digestion==== | |
| - | + | BBa_K342001(PhaC) was digested by XbaI and PstI.<br />And BBa_B0034(RBS) was digested by SpeI and PstI (with three samples). | |
| - | + | ||
PhaC (BBa_K342001) | PhaC (BBa_K342001) | ||
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
| Line 32: | Line 31: | ||
|20 ul | |20 ul | ||
|} | |} | ||
| - | + | ||
| + | |||
RBS (BBa_B0034) | RBS (BBa_B0034) | ||
| - | + | N0. 1 | |
| - | N0.1 | + | |
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
|- | |- | ||
| Line 56: | Line 55: | ||
|25 ul | |25 ul | ||
|} | |} | ||
| - | + | ||
| - | N0.2 | + | |
| + | N0. 2 | ||
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
|- | |- | ||
| Line 78: | Line 78: | ||
|25 ul | |25 ul | ||
|} | |} | ||
| - | |||
| - | N0.3 | + | |
| + | N0. 3 | ||
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
|- | |- | ||
| Line 101: | Line 101: | ||
|25 ul | |25 ul | ||
|} | |} | ||
| - | |||
| - | ==Gel extraction== | + | ====Electrophoresis==== |
| - | < | + | We confirmed success of digestion by electrophoresis.<br/>The DNA was extracted from TBE gel and we got DNA solution. |
| + | |||
| + | ====Gel extraction==== | ||
| + | The digestion product was extracted. Used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution. | ||
| + | |||
| + | </div></div> | ||
| + | |||
| + | <div class="hokkaidou-notebook-daily"> | ||
| + | ===August 28th=== | ||
| + | <div class="hokkaidou-section"> | ||
| + | ====Digestion==== | ||
| + | Digestion to divide PhaA and PhaB with XbaI and PstI.<br />I digested PhaC(BBa_K342001) with these restriction sites and also XhoI to divide pSB1C3 into pieces, on which PhaC is, that is because the length of pSB1C3 is nearly PhaC.<br />And we digested PhaC (BBa_K342001) and pSB1C3 by XbaI and SpeI.<br /><br /> | ||
| + | PhaA | ||
| + | {|class="hokkaidou-table-digestion" | ||
| + | |- | ||
| + | |DNA solution (125 ng/ul) | ||
| + | |6.6 ul | ||
| + | |- | ||
| + | |XbaI | ||
| + | |1 ul | ||
| + | |- | ||
| + | |PstI | ||
| + | |1 ul | ||
| + | |- | ||
| + | |10xM buffer | ||
| + | |2 ul | ||
| + | |- | ||
| + | |DW | ||
| + | |9.4 ul | ||
| + | |- | ||
| + | |Total | ||
| + | |20 ul | ||
| + | |} | ||
| + | |||
| + | |||
| + | PhaB | ||
| + | {|class="hokkaidou-table-digestion" | ||
| + | |- | ||
| + | |DNA solution (125 ng/ul) | ||
| + | |4 ul | ||
| + | |- | ||
| + | |XbaI | ||
| + | |1 ul | ||
| + | |- | ||
| + | |PstI | ||
| + | |1 ul | ||
| + | |- | ||
| + | |10xM buffer | ||
| + | |2 ul | ||
| + | |- | ||
| + | |DW | ||
| + | |12 ul | ||
| + | |- | ||
| + | |Total | ||
| + | |20 ul | ||
| + | |} | ||
| + | |||
| + | |||
| + | PhaC(BBa_K342001) | ||
| + | {|class="hokkaidou-table-digestion" | ||
| + | |- | ||
| + | |DNA solution (125 ng/ul) | ||
| + | |10 ul | ||
| + | |- | ||
| + | |XbaI | ||
| + | |1 ul | ||
| + | |- | ||
| + | |PstI | ||
| + | |1 ul | ||
| + | |- | ||
| + | |XhoI | ||
| + | |5.1 ul | ||
| + | |- | ||
| + | |10xM buffer | ||
| + | |2 ul | ||
| + | |- | ||
| + | |DW | ||
| + | |0.9 ul | ||
| + | |- | ||
| + | |Total | ||
| + | |20 ul | ||
| + | |} | ||
| + | |||
| + | ====Electrophoresis==== | ||
| + | We confirmed success of digestion by electrophoresis.<br/>The DNA was extracted from TBE gel and we got DNA solution. | ||
| + | |||
| + | ====Gel extraction==== | ||
Gel extraction for digestion product. Used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution. | Gel extraction for digestion product. Used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution. | ||
| - | </ | + | </div></div> |
| + | |||
| + | <div class="hokkaidou-notebook-daily"> | ||
| + | ===August 29th=== | ||
| + | <div class="hokkaidou-section"> | ||
| + | ====PHB polymer ethanolysis==== | ||
| + | We did ethanolysisãof PHB polymer for 4 hrs with sample 2~8. | ||
| + | |||
| + | ====Preparation for GC/MS==== | ||
| + | We did the preparation for GC/MS with sample 2~8. | ||
| + | |||
| + | ====PCR==== | ||
| + | We multiplied pSB1C3 by PCR. | ||
| + | Used two different DNA polymerase, KOD-Plus-Neo and KAPA Taq. | ||
| + | {|class="hokkaidou-table-pcr-reagent" | ||
| + | |Solution | ||
| + | |Volume (ul) | ||
| + | |- | ||
| + | |DNA | ||
| + | |1 | ||
| + | |- | ||
| + | |Suffix-EX | ||
| + | |1 | ||
| + | |- | ||
| + | |Prefix-PS | ||
| + | |1 | ||
| + | |- | ||
| + | |MgSO4 | ||
| + | |3 | ||
| + | |- | ||
| + | |dNTP | ||
| + | |5 | ||
| + | |- | ||
| + | |10x KOD-Plus-Neo Buffer | ||
| + | |5 | ||
| + | |- | ||
| + | |KOD-Plus-Neo | ||
| + | |1 | ||
| + | |- | ||
| + | |DW | ||
| + | |33 | ||
| + | |- | ||
| + | |Total | ||
| + | |50 | ||
| + | |} | ||
| + | |||
| + | |||
| + | {|class="hokkaidou-table-pcr-time" | ||
| + | |- | ||
| + | |Number | ||
| + | |Degree | ||
| + | |Second | ||
| + | |- | ||
| + | |1 | ||
| + | |94 | ||
| + | |120 | ||
| + | |- | ||
| + | |2 | ||
| + | |98 | ||
| + | |10 | ||
| + | |- | ||
| + | |3 | ||
| + | |74 | ||
| + | |2 | ||
| + | |- | ||
| + | |4 | ||
| + | |98 | ||
| + | |10 | ||
| + | |- | ||
| + | |5 | ||
| + | |72 | ||
| + | |120 | ||
| + | |- | ||
| + | |6 | ||
| + | |98 | ||
| + | |10 | ||
| + | |- | ||
| + | |7 | ||
| + | |70 | ||
| + | |120 | ||
| + | |- | ||
| + | |8 | ||
| + | |98 | ||
| + | |10 | ||
| + | |- | ||
| + | |9 | ||
| + | |68 | ||
| + | |120 | ||
| + | |- | ||
| + | |10 | ||
| + | |68 | ||
| + | |420 | ||
| + | |- | ||
| + | |11 | ||
| + | |4 | ||
| + | |HOLD | ||
| + | |} | ||
| + | Cycle1 : 2~3 x 5 | ||
| + | Cycle2 : 4~5 x 5 | ||
| + | Cycle3 : 6~7 x 5 | ||
| + | Cycle4 : 8~9 x 30 | ||
| + | |||
| + | |||
| + | {|class="hokkaidou-table-pcr-reagent" | ||
| + | |Solution | ||
| + | |Volume (ul) | ||
| + | |- | ||
| + | |DNA | ||
| + | |1 | ||
| + | |- | ||
| + | |Suffix-EX (10 uM) | ||
| + | |2 | ||
| + | |- | ||
| + | |Prefix-PS (10 uM) | ||
| + | |2 | ||
| + | |- | ||
| + | |KAPA Taq | ||
| + | |25 | ||
| + | |- | ||
| + | |DW | ||
| + | |20 | ||
| + | |- | ||
| + | |Total | ||
| + | |50 | ||
| + | |} | ||
| + | |||
| + | {|class="hokkaidou-table-pcr-time" | ||
| + | |- | ||
| + | |Number | ||
| + | |Degree | ||
| + | |Second | ||
| + | |- | ||
| + | |1 | ||
| + | |95 | ||
| + | |120 | ||
| + | |- | ||
| + | |2 | ||
| + | |95 | ||
| + | |30 | ||
| + | |- | ||
| + | |3 | ||
| + | |63.7 | ||
| + | |30 | ||
| + | |- | ||
| + | |4 | ||
| + | |72 | ||
| + | |180 | ||
| + | |- | ||
| + | |5 | ||
| + | |4 | ||
| + | |HOLD | ||
| + | |} | ||
| + | Cycle:2~4 x 35 | ||
| + | |||
| + | </div></div> | ||
| + | |||
| + | <div class="hokkaidou-notebook-daily"> | ||
| + | ===August 30th=== | ||
| + | <div class="hokkaidou-section"> | ||
| + | ====Ethanol precipitation==== | ||
| + | Diegested phaA by XbaI and SpeI and RBS by SpeI were condensed by Ethanol precipitation. | ||
| + | |||
| + | ====Ligation==== | ||
| + | PhaA and RBS, PhaB and RBS, PhaB and pSB1C3 were ligated each other.<br />And the DNA were transformed into bacteria. | ||
| + | |||
| + | ====Colony PCR==== | ||
| + | The length of PhaB on pSB1C3 was confirmed by colony PCR.<br />The result showed PhaB and pSB1C3 didn't ligate correctly. | ||
| + | |||
| + | ====Liquid Culture==== | ||
| + | Incubation of bacteria holds RBS (BBa_B0034) - PhaC (K342001) was started. | ||
| + | |||
| + | </div></div> | ||
| + | |||
| + | <div class="hokkaidou-notebook-daily"> | ||
| + | ===August 31st=== | ||
| + | <div class="hokkaidou-section"> | ||
| + | ====Colony PCR==== | ||
| + | We confirmed the length of the three constructs that transformed at August 30th.<br />The result showed RBS and PhaA were ligated correctly.<br />So the incubation was started. | ||
| + | [[image:HokkaidoU 120831 RBS phaA coloP edit (2).jpg|thumb|center|450px]] | ||
| + | |||
| + | ====Plasmid extraction==== | ||
| + | Plasmid of RBS-PhaC were extracted.<br />And then we got 50ul DNA solution. | ||
| + | |||
| + | ====Liquid culture==== | ||
| + | Incubation of bacteria holds dT (BBa_B0015) was started. | ||
| + | |||
| + | </div></div> | ||
| + | |||
| + | <div class="hokkaidou-notebook-daily"> | ||
| + | ===September 1st=== | ||
| + | <div class="hokkaidou-section"> | ||
| + | |||
| + | ====Plasmid extraction==== | ||
| + | Plasmids of RBS-PhaA and dT (BBa_B0015) were extracted.<br />And then we got 50ul DNA solution. | ||
| + | [[image:HokkaidoU 120901 dT RBS-PhaA RBS-PhaC mini-prepç£ç© edit.jpg|thumb|center|600px|center|Fig. Extracted plasmids of dT and RBS-PhaA on pSB1A2]]<br /> | ||
| + | 1: dT on pSB1AK3 (About 3.3kbp)<br /> | ||
| + | 2 to 4: RBS-PhaA on pSB1A2 (About 3.2kbp)<br /> | ||
| + | 5 to 7: RBS-PhaC on pSB1A2 (About 4.1kbp)<br />We thought sample 4 is not ideal plasmid and trashed it. | ||
| + | |||
| + | ====Digestion==== | ||
| + | RBS-PhaA was digested by XbaI and SpeI restriction site to ligate with RBS-PhaC digested by SpeI site. | ||
| + | [[image:HokkaidoU 120901 RBS-PhaA RBS-PhaC digestion.jpg]] | ||
| + | *1 and 2 is digested RBS-PhaA on pSB1A2. | ||
| + | *Upper fragment is vector, pSB1A2. | ||
| + | *Lower one is an objective fragment, RBS-PhaA (About 1.2kbp). | ||
| + | *And PhaB and pSB1C3 were digested with XbaI and SpeI site.<br/>We decided to try ligation PhaB with pSB1C3 again. | ||
| + | |||
| + | ====Electrophoresis==== | ||
| + | We confirmed success of digestion by electrophoresis.<br/>The DNA was extracted from TBE gel and we got DNA solution. | ||
| + | </div></div> | ||
| + | |||
| + | <div class="hokkaidou-notebook-daily"> | ||
| + | |||
| + | ===September 2nd=== | ||
| + | <div class="hokkaidou-section"> | ||
| + | |||
| + | ====Ethanol precipitation==== | ||
| + | The digested DNAs, RBS-PhaA (No. 1), RBS-PhaA (No. 2), RBS-PhaC on pSB1A2, PhaB and pSB1C3 were concentrated by Ethanol precipitation. | ||
| + | |||
| + | ====Ligation==== | ||
| + | RBS-PhaA (No. 1 and No. 2) was ligated with RBS-PhaC on pSB1A2.<br/> | ||
| + | And PhaB was taken in pSB1C3. | ||
| + | |||
| + | ====Transformation==== | ||
| + | These ligated DNAs transformed into E. coli (strain: DH5α).<br/> | ||
| + | And then we spread fungus liquid added LB on LB plates include antibiotics. | ||
</div></div> | </div></div> | ||
Latest revision as of 04:02, 27 September 2012
Contents |
August 27th
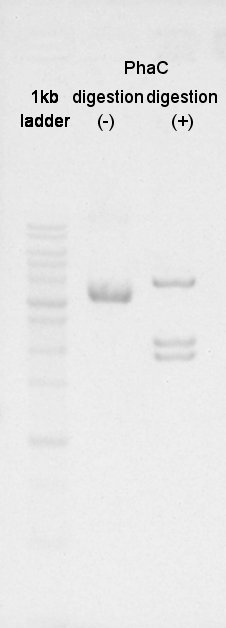
Digestion
BBa_K342001(PhaC) was digested by XbaI and PstI.
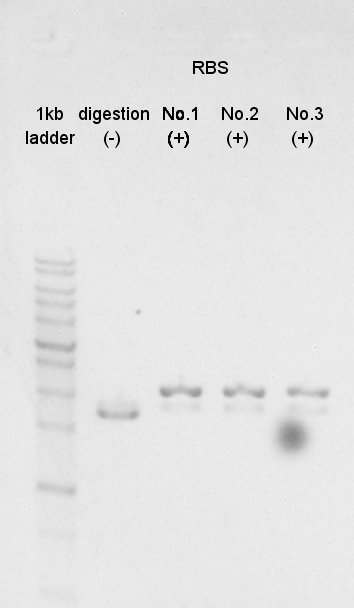
And BBa_B0034(RBS) was digested by SpeI and PstI (with three samples).
PhaC (BBa_K342001)
| DNA solution (100 ng/ul) | 12.5 ul |
| XbaI | 1 ul |
| PstI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 3.5 ul |
| Total | 20 ul |
RBS (BBa_B0034)
N0. 1
| DNA solution (20.3 ng/ul) | 14.3 ul |
| SpeI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2.5 ul |
| DW | 0.2 ul |
| Total | 25 ul |
N0. 2
| DNA solution (15.6 ng/ul) | 18.6 ul |
| SpeI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2.5 ul |
| DW | 1.9 ul |
| Total | 25 ul |
N0. 3
| DNA solution (16.9 ng/ul) | 17.2 ul |
| SpeI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2.5 ul |
| DW | 3.3 ul |
| Total | 25 ul |
Electrophoresis
We confirmed success of digestion by electrophoresis.
The DNA was extracted from TBE gel and we got DNA solution.
Gel extraction
The digestion product was extracted. Used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution.
August 28th
Digestion
Digestion to divide PhaA and PhaB with XbaI and PstI.
I digested PhaC(BBa_K342001) with these restriction sites and also XhoI to divide pSB1C3 into pieces, on which PhaC is, that is because the length of pSB1C3 is nearly PhaC.
And we digested PhaC (BBa_K342001) and pSB1C3 by XbaI and SpeI.
PhaA
| DNA solution (125 ng/ul) | 6.6 ul |
| XbaI | 1 ul |
| PstI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 9.4 ul |
| Total | 20 ul |
PhaB
| DNA solution (125 ng/ul) | 4 ul |
| XbaI | 1 ul |
| PstI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 12 ul |
| Total | 20 ul |
PhaC(BBa_K342001)
| DNA solution (125 ng/ul) | 10 ul |
| XbaI | 1 ul |
| PstI | 1 ul |
| XhoI | 5.1 ul |
| 10xM buffer | 2 ul |
| DW | 0.9 ul |
| Total | 20 ul |
Electrophoresis
We confirmed success of digestion by electrophoresis.
The DNA was extracted from TBE gel and we got DNA solution.
Gel extraction
Gel extraction for digestion product. Used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution.
August 29th
PHB polymer ethanolysis
We did ethanolysisãof PHB polymer for 4 hrs with sample 2~8.
Preparation for GC/MS
We did the preparation for GC/MS with sample 2~8.
PCR
We multiplied pSB1C3 by PCR. Used two different DNA polymerase, KOD-Plus-Neo and KAPA Taq.
| Solution | Volume (ul) |
| DNA | 1 |
| Suffix-EX | 1 |
| Prefix-PS | 1 |
| MgSO4 | 3 |
| dNTP | 5 |
| 10x KOD-Plus-Neo Buffer | 5 |
| KOD-Plus-Neo | 1 |
| DW | 33 |
| Total | 50 |
| Number | Degree | Second |
| 1 | 94 | 120 |
| 2 | 98 | 10 |
| 3 | 74 | 2 |
| 4 | 98 | 10 |
| 5 | 72 | 120 |
| 6 | 98 | 10 |
| 7 | 70 | 120 |
| 8 | 98 | 10 |
| 9 | 68 | 120 |
| 10 | 68 | 420 |
| 11 | 4 | HOLD |
Cycle1 : 2~3 x 5 Cycle2 : 4~5 x 5 Cycle3 : 6~7 x 5 Cycle4 : 8~9 x 30
| Solution | Volume (ul) |
| DNA | 1 |
| Suffix-EX (10 uM) | 2 |
| Prefix-PS (10 uM) | 2 |
| KAPA Taq | 25 |
| DW | 20 |
| Total | 50 |
| Number | Degree | Second |
| 1 | 95 | 120 |
| 2 | 95 | 30 |
| 3 | 63.7 | 30 |
| 4 | 72 | 180 |
| 5 | 4 | HOLD |
Cycle:2~4 x 35
August 30th
Ethanol precipitation
Diegested phaA by XbaI and SpeI and RBS by SpeI were condensed by Ethanol precipitation.
Ligation
PhaA and RBS, PhaB and RBS, PhaB and pSB1C3 were ligated each other.
And the DNA were transformed into bacteria.
Colony PCR
The length of PhaB on pSB1C3 was confirmed by colony PCR.
The result showed PhaB and pSB1C3 didn't ligate correctly.
Liquid Culture
Incubation of bacteria holds RBS (BBa_B0034) - PhaC (K342001) was started.
August 31st
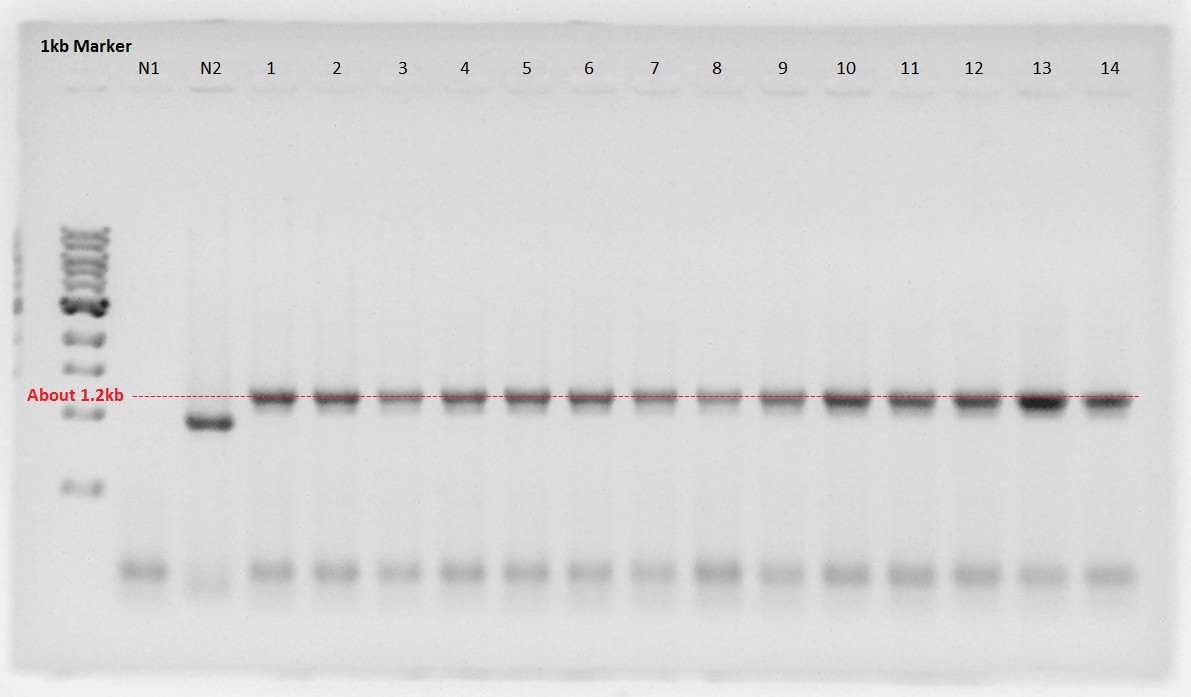
Colony PCR
We confirmed the length of the three constructs that transformed at August 30th.
The result showed RBS and PhaA were ligated correctly.
So the incubation was started.
Plasmid extraction
Plasmid of RBS-PhaC were extracted.
And then we got 50ul DNA solution.
Liquid culture
Incubation of bacteria holds dT (BBa_B0015) was started.
September 1st
Plasmid extraction
Plasmids of RBS-PhaA and dT (BBa_B0015) were extracted.
And then we got 50ul DNA solution.
1: dT on pSB1AK3 (About 3.3kbp)
2 to 4: RBS-PhaA on pSB1A2 (About 3.2kbp)
5 to 7: RBS-PhaC on pSB1A2 (About 4.1kbp)
We thought sample 4 is not ideal plasmid and trashed it.
Digestion
RBS-PhaA was digested by XbaI and SpeI restriction site to ligate with RBS-PhaC digested by SpeI site.

- 1 and 2 is digested RBS-PhaA on pSB1A2.
- Upper fragment is vector, pSB1A2.
- Lower one is an objective fragment, RBS-PhaA (About 1.2kbp).
- And PhaB and pSB1C3 were digested with XbaI and SpeI site.
We decided to try ligation PhaB with pSB1C3 again.
Electrophoresis
We confirmed success of digestion by electrophoresis.
The DNA was extracted from TBE gel and we got DNA solution.
September 2nd
Ethanol precipitation
The digested DNAs, RBS-PhaA (No. 1), RBS-PhaA (No. 2), RBS-PhaC on pSB1A2, PhaB and pSB1C3 were concentrated by Ethanol precipitation.
Ligation
RBS-PhaA (No. 1 and No. 2) was ligated with RBS-PhaC on pSB1A2.
And PhaB was taken in pSB1C3.
Transformation
These ligated DNAs transformed into E. coli (strain: DH5α).
And then we spread fungus liquid added LB on LB plates include antibiotics.
 "
"