Team:HokkaidoU Japan/Notebook/aggregation Week 3
From 2012.igem.org
| (56 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
<!-- DO NOT EDIT OVER THIS LINE @iTakeshi --> | <!-- DO NOT EDIT OVER THIS LINE @iTakeshi --> | ||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==July 16th== | + | ===July 16th=== |
| - | <div> | + | <div class="hokkaidou-section"> |
Ag43, dT | Ag43, dT | ||
| - | ===Electrophoresis=== | + | ====Electrophoresis==== |
| - | + | Results of digestion at 15th. | |
| - | Results of digestion | + | |
[[image:HokkaidoU2012 120716 Ag43 on pSB1C3 digestion with S&P-1.jpg|thumb|Digestion result image]] | [[image:HokkaidoU2012 120716 Ag43 on pSB1C3 digestion with S&P-1.jpg|thumb|Digestion result image]] | ||
| - | Product:Ag43(K346007)=3120bp and 2070bp | + | Product:Ag43(K346007)=3120bp and 2070bp<br />pT7-RBS on pSB1K3=2247bp |
| - | We confirmed there are some | + | <br /> |
| - | + | We confirmed there are some kinds of restriction enzyme site in K346007 (digested with SpeI, PstI), and pT7-RBS on pSB1K3 was successfully digested with EcoRI and PstI.ãBalance between d+(EcoRI) and d+(E&P:cut with EcoRI and PstI) is about 80bp. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | === | + | ====Gel extraction==== |
| - | + | Gel extraction for digestion products. Used FastGene Gel&PCR extraction kit(NipponGenetics) andãgot 50 ul of DNA solution. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ====Ethanol precipitation==== | |
| + | Ethanol precipitation for digested and extracted products. | ||
| + | #Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. | ||
| + | #Centrifuged at 15000 rpm, 10 min at 4C. | ||
| + | #Removed supernatant and added 220 ul of 70% ethanol. | ||
| + | #Centrifuged at 15000 rpm, 10 min at 4C. | ||
| + | #Removed supernatant and air drying at room temperature then added 5 ul of DW. | ||
| - | |||
| - | ===PCR=== | + | dT(B0015) would be amplifiedãincorrectly and we couldn't get enough digestion product. So we tried another DNA amplification method: PCR then digested. |
| - | + | ====PCR==== | |
PCR for dT(B0015) | PCR for dT(B0015) | ||
| Line 43: | Line 34: | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |1 ul |
|- | |- | ||
|KOD-Plus-NEO(Taq polymerase) | |KOD-Plus-NEO(Taq polymerase) | ||
| - | | | + | |1 ul |
|- | |- | ||
|dNTP | |dNTP | ||
| - | | | + | |5 ul |
|- | |- | ||
|MgSO4 | |MgSO4 | ||
| - | | | + | |3 ul |
|- | |- | ||
|KOD-Plus-NEO Buffer | |KOD-Plus-NEO Buffer | ||
| - | | | + | |5 ul |
|- | |- | ||
|Forward Primer(100bp_up forward primer) | |Forward Primer(100bp_up forward primer) | ||
| - | | | + | |1 ul |
|- | |- | ||
|Reverse Primer(200bp_down Reverse primer) | |Reverse Primer(200bp_down Reverse primer) | ||
| - | | | + | |1 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |33 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |50 ul |
|} | |} | ||
| Line 98: | Line 89: | ||
|} | |} | ||
Cycle:2~4 x 45 | Cycle:2~4 x 45 | ||
| - | |||
[[image:HokkaidoU2012 120716-dt-ethandpcr.jpg|thumb|PCR result image]] | [[image:HokkaidoU2012 120716-dt-ethandpcr.jpg|thumb|PCR result image]] | ||
| - | We migrated B0015 | + | We migrated B0015 of plasmid extraction product, digestion product, and PCR product. |
| - | + | dT done PCR would have 429bp(100bp(added by forward primer) + 129bp(dT) + 200bp(added by reverse primer)). Most bright and thick band in this image has about 300~400 bp. We thought dT was amplified successfully. | |
| - | + | ||
| + | ====Gel extraction==== | ||
| + | Gel extraction for digestion products. We used FastGene Gel&PCR extraction kit(NipponGenetics). | ||
| + | Got 50 ul of DNA solution. | ||
| - | === | + | ====Digestion==== |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
'''Digestion for dT which amplified with PCR.''' | '''Digestion for dT which amplified with PCR.''' | ||
Digested with XbaI and PstI. | Digested with XbaI and PstI. | ||
| - | + | <br /> | |
dT | dT | ||
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |5 ul |
|- | |- | ||
|XbaI | |XbaI | ||
| - | | | + | |1 ul |
|- | |- | ||
|PstI | |PstI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xM buffer | |10xM buffer | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |11 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |20 ul |
|} | |} | ||
| - | |||
| - | |||
Ag43 | Ag43 | ||
| - | |||
'''Digestion result of Ag43 was incorrect'''. We digested Ag43 once more time. | '''Digestion result of Ag43 was incorrect'''. We digested Ag43 once more time. | ||
| - | + | ====Digestion==== | |
| - | ===Digestion=== | + | |
| - | + | ||
Digestion for Ag43 with SpeI and PstI. | Digestion for Ag43 with SpeI and PstI. | ||
| Line 155: | Line 135: | ||
|- | |- | ||
|Ag43 DNA solution | |Ag43 DNA solution | ||
| - | | | + | |9 ul |
|- | |- | ||
|SpeI | |SpeI | ||
| - | | | + | |1 ul |
|- | |- | ||
|PstI | |PstI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |7 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |20 ul |
|} | |} | ||
| Line 178: | Line 158: | ||
There are same results with digestion result of recent. '''We thought PstI would cut different site'''. | There are same results with digestion result of recent. '''We thought PstI would cut different site'''. | ||
What is this 500bp fragment???? | What is this 500bp fragment???? | ||
| - | |||
| - | ===Gel extraction=== | + | ====Gel extraction==== |
| - | + | Gel extraction for digestion products. We used FastGene Gel&PCR extraction kit(NipponGenetics). | |
| - | Gel | + | |
Got 50ul of DNA solution. | Got 50ul of DNA solution. | ||
| - | |||
| - | + | ====Liquid Culture==== | |
| - | + | ||
| - | ===Liquid Culture=== | + | |
| - | + | ||
Liquid culture for single colony isolated pT7-RBS on pSB1C3 and Ag43-dT on pSB1T3. | Liquid culture for single colony isolated pT7-RBS on pSB1C3 and Ag43-dT on pSB1T3. | ||
| - | '''Ag43-dT on pSB1T3 | + | '''Colonies which have Ag43-dT on pSB1T3 were closely existed so we would picked up two or more colonies.''' |
| - | #Picked up one (or tow?) colony from single colony isolated | + | #Picked up one (or tow?) colony from single colony isolated platesãby platinum loop. |
| - | #Dipped into | + | #Dipped into 2 ml of LBC and LBT. |
| - | # | + | #Incuvated. |
| - | </ | + | |
| + | <br style="line-height: 0; clear: both;" /> | ||
| + | |||
</div></div> | </div></div> | ||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==July 17th== | + | |
| - | <div | + | ===July 17th=== |
| - | + | <div class="hokkaidou-section"> | |
Ag43-dT on pSB1T3 and pT7-RBS on pSB1C3 | Ag43-dT on pSB1T3 and pT7-RBS on pSB1C3 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| + | ====Plasmid extraction==== | ||
| + | Plasmid extraction for Ag43-dT on pSB1T3 and pT7-RBS on pSB1C3 liquid culture products incuvated from yesterday(16th). | ||
| + | We used FastGene Plasmid Mini Kit(Nippon Genetics) and got 50 ul of DNA solutions. | ||
| + | |||
| + | ====Electrophoresis==== | ||
| + | Electrophoresis for digestion product of K346007(Ag43) with EcoRI and SpeI as a reference to confirm SpeI cuts correct site or not and PstI didn't work correctly(in past experiments). And plasmid extraction products of Ag43-dT on pSB1T3 and pT7-RBS on pSB1C3 also migrated. | ||
If digestion were succeeded, there are two bands would be seen: about 3120bp and 2070bp. | If digestion were succeeded, there are two bands would be seen: about 3120bp and 2070bp. | ||
| - | And if ligation | + | And if ligation and transformation, plasmid extraction were succeeded, there would be existed two bands in each lanes: about 5000bp and 2000bp. |
| - | + | [[image:HokkaidoU2012 120717 Ag43-Ag43d+(E&S)-(Ag43-dT on pSB1T3)-(pT7-RBS on pSB1C3).jpg|thumb|Digestion and plasmid extraction result image]] | |
| - | [[image:HokkaidoU2012 120717 Ag43-Ag43d+(E&S)-(Ag43-dT on pSB1T3)-(pT7-RBS on pSB1C3).jpg|thumb|Digestion and | + | From this result we confirmed Ag43 was successfully digested with EcoRI and SpeI, and there were no other extra bands which had been existed double digested with EcoRI & PstI and SpeI & PstI. '''would PstI not work correctly?''' |
| - | + | ||
| - | + | ||
| - | + | ||
'''We planed to use dT as vector and Ag43 as Insert.''' A problem is if dT on pSB1AK3 is used as vector and Ag43 used as insert, we can't select correct Ag43 band in gel extraction phase because DNA bp of Ag43 is nearly same as pSB1AK3. Thus we tried to use dT on pSB1T3 as vector and Ag43 on pSB1C3 as vector and ligate with standard assembly. | '''We planed to use dT as vector and Ag43 as Insert.''' A problem is if dT on pSB1AK3 is used as vector and Ag43 used as insert, we can't select correct Ag43 band in gel extraction phase because DNA bp of Ag43 is nearly same as pSB1AK3. Thus we tried to use dT on pSB1T3 as vector and Ag43 on pSB1C3 as vector and ligate with standard assembly. | ||
| - | + | About plasmid extraction products, in Ag43-dT on pSB1T3, there were two plasmid DNA bands about 8000bp and 3000bp in one lane. We thought '''which means we failed single colony isolation then resuspended two another E.coli colonies''' another ligated DNA were transformed. | |
| - | About | + | |
In pT7-RBS on pSB1C3, we needed about 2000bp plasmid DNA and there were about 1500bp of DNA. Plasmid DNA migrates more far than linear DNA so we thought we got correct DNA. | In pT7-RBS on pSB1C3, we needed about 2000bp plasmid DNA and there were about 1500bp of DNA. Plasmid DNA migrates more far than linear DNA so we thought we got correct DNA. | ||
| + | To confirm plasmid extraction products were really ligated correct DNA fragments, first we extracted Ag43-dT on pSB1T3(low bp band) from TBE gel and digested with EcoRI and PstI. | ||
| - | To | + | '''dT Vector plasmid change''' |
| - | + | To use dT as a vector, we needed to change plasmid backbone pAB1AK3 to pSB1T3. | |
| - | + | ====Digestion==== | |
| - | + | ||
| - | + | ||
| - | ==Digestion== | + | |
| - | + | ||
Digestion to change the plasmid backbone. | Digestion to change the plasmid backbone. | ||
| - | Used DNA solution as PCR product( | + | Used DNA solution as PCR product(did at 16th) and digestioned pSB1T3 were already exist.<br /> |
| - | + | ||
| - | + | ||
| - | + | ||
| + | Digestion mix<br /><br /> | ||
double digestion(EcoRI and PstI) | double digestion(EcoRI and PstI) | ||
| Line 249: | Line 213: | ||
|- | |- | ||
|dT PCR product | |dT PCR product | ||
| - | | | + | |1 ul |
|- | |- | ||
|EcoRI | |EcoRI | ||
| - | | | + | |1 ul |
|- | |- | ||
|PstI | |PstI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |15 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |20 ul |
|} | |} | ||
| Line 272: | Line 236: | ||
|- | |- | ||
|dT PCR product | |dT PCR product | ||
| - | | | + | |1 ul |
|- | |- | ||
|EcoRI | |EcoRI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |16 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |20 ul |
|} | |} | ||
| Line 292: | Line 256: | ||
|- | |- | ||
|dT PCR product | |dT PCR product | ||
| - | | | + | |1 ul |
|- | |- | ||
|PstI | |PstI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |16 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |20 ul |
|} | |} | ||
| Line 312: | Line 276: | ||
We couldn't confirm digestion results. | We couldn't confirm digestion results. | ||
| + | pT7-RBS on pSB1C3 and Ag43-dT on pSB1T3 | ||
| - | + | ====Electrophoresis and gel extraction==== | |
| - | + | [[image:HokkaidoU2012 120717-ag43-dt-psb1t3.jpg|thumb|plasmid extraction result]] | |
| - | + | '''Gel extraction for Ag43-dT on pSB1T3.'''<br /> | |
| - | + | ||
| - | ==Electrophoresis and | + | |
| - | + | ||
| - | [[image:HokkaidoU2012 120717-ag43-dt-psb1t3.jpg|thumb| | + | |
| - | '''Gel extraction for Ag43-dT on pSB1T3.''' | + | |
We cut low bp band (see image below). | We cut low bp band (see image below). | ||
Gel Extraction for digestion products. We used FastGene Gel&PCR extraction kit(NipponGenetics). | Gel Extraction for digestion products. We used FastGene Gel&PCR extraction kit(NipponGenetics). | ||
| - | Got | + | Got 50 ul of DNA solution. |
| + | ====Digestion==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
Digestion Ag43-dT on pSB1T3 and pT7-RBS on pSB1C3 to confirm ligation was succeeded or not. | Digestion Ag43-dT on pSB1T3 and pT7-RBS on pSB1C3 to confirm ligation was succeeded or not. | ||
| + | <br /><br /> | ||
| - | + | '''Ag43-dT on pSB1T3(30 ng/ul)''' | |
| - | '''Ag43-dT on pSB1T3( | + | |
| - | + | ||
Control 1(EcoRI only) | Control 1(EcoRI only) | ||
| Line 340: | Line 296: | ||
|- | |- | ||
|Ag43-dT DNA solution | |Ag43-dT DNA solution | ||
| - | |3. | + | |3.3 ul |
|- | |- | ||
|EcoRI | |EcoRI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | |14. | + | |14.7 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |21 ul |
|} | |} | ||
| Line 360: | Line 316: | ||
|- | |- | ||
|Ag43-dT DNA solution | |Ag43-dT DNA solution | ||
| - | |3. | + | |3.3 ul |
|- | |- | ||
|PstI | |PstI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | |14. | + | |14.7 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |21 ul |
|} | |} | ||
| Line 380: | Line 336: | ||
|- | |- | ||
|Ag43-dT DNA solution | |Ag43-dT DNA solution | ||
| - | |3. | + | |3.3 ul |
|- | |- | ||
|EcoRI | |EcoRI | ||
| - | | | + | |1 ul |
|- | |- | ||
|PstI | |PstI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | |13. | + | |13.7 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |21 ul |
|} | |} | ||
| - | '''pT7-RBS on pSB1C3( | + | '''pT7-RBS on pSB1C3(40 ng/ul)''' |
| - | + | <br /><br /> | |
Control 1(EcoRI only) | Control 1(EcoRI only) | ||
| Line 406: | Line 362: | ||
|- | |- | ||
|pT7-RBS DNA solution | |pT7-RBS DNA solution | ||
| - | |2. | + | |2.5 ul |
|- | |- | ||
|EcoRI | |EcoRI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | |15. | + | |15.5 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |21 ul |
|} | |} | ||
| Line 426: | Line 382: | ||
|- | |- | ||
|pT7-RBS DNA solution | |pT7-RBS DNA solution | ||
| - | |2. | + | |2.5 ul |
|- | |- | ||
|PstI | |PstI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | |15. | + | |15.5 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |21 ul |
|} | |} | ||
| Line 446: | Line 402: | ||
|- | |- | ||
|pT7-RBS DNA solution | |pT7-RBS DNA solution | ||
| - | |2. | + | |2.5 ul |
|- | |- | ||
|EcoRI | |EcoRI | ||
| - | | | + | |1 ul |
|- | |- | ||
|PstI | |PstI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | |14. | + | |14.5 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |21 ul |
|} | |} | ||
| Line 467: | Line 423: | ||
[[image:HokkaidoU2012 120717 Ag43 dT(d-,d+E,d+P.jpg|thumb|Digestion result]] | [[image:HokkaidoU2012 120717 Ag43 dT(d-,d+E,d+P.jpg|thumb|Digestion result]] | ||
| - | + | From this digestion results, | |
*Cut with EcoRI,PstI and E&P(EcoRI and PstI) showed different base pair bands compared with d-(digestion minus). | *Cut with EcoRI,PstI and E&P(EcoRI and PstI) showed different base pair bands compared with d-(digestion minus). | ||
*Cut with E&P showed two DNA bands: about 2000bp and 500~1000 bp. | *Cut with E&P showed two DNA bands: about 2000bp and 500~1000 bp. | ||
*All of digestion products existed lower place than correct band.Correct DNA would show about 5000bp(EcoRI and PstI), 3000bp and 2000bp (E&P). | *All of digestion products existed lower place than correct band.Correct DNA would show about 5000bp(EcoRI and PstI), 3000bp and 2000bp (E&P). | ||
| - | </ | + | |
| + | <br style="line-height: 0; clear: both;" /> | ||
| + | |||
</div></div> | </div></div> | ||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==July 18th== | + | ===July 18th=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | + | '''We decided change plasmid backbone of dT pSB1AK3 to pSB1T3.'''<br /> | |
| - | + | If we cut Ag43-dT on pSB1AK3 with EcoRI and PstI to confirm insert DNA, DNA fragment base pair resemble each other(about 3000bp), so then we can't identify them. | |
| - | '''We decided change plasmid backbone of dT pSB1AK3 to pSB1T3.''' | + | |
| - | If we cut Ag43-dT on pSB1AK3 with EcoRI and PstI to confirm insert DNA, DNA fragment base pair resemble each other(about 3000bp)then we can't | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | ====digestion==== | ||
'''Digestion to confirm what kind of restriction enzyme sites Ag43(K346007) has.''' | '''Digestion to confirm what kind of restriction enzyme sites Ag43(K346007) has.''' | ||
| - | + | <br /><br /> | |
EcoRI | EcoRI | ||
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |1 ul |
|- | |- | ||
|EcoRI | |EcoRI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |1 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |7 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |10 ul |
|} | |} | ||
| Line 512: | Line 465: | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |1 ul |
|- | |- | ||
|XbaI | |XbaI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xM buffer | |10xM buffer | ||
| - | | | + | |1 ul |
|- | |- | ||
|BSA | |BSA | ||
| - | | | + | |1 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |6 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |10 ul |
|} | |} | ||
| Line 535: | Line 488: | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |1 ul |
|- | |- | ||
|SpeI | |SpeI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |1 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |7 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |10 ul |
|} | |} | ||
| Line 555: | Line 508: | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |1 ul |
|- | |- | ||
|PstI | |PstI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |1 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |7 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |10 ul |
|} | |} | ||
[[image:HokkaidoU2012 120718-k346007-digestion print.jpg|thumb|Digestion result]] | [[image:HokkaidoU2012 120718-k346007-digestion print.jpg|thumb|Digestion result]] | ||
| - | + | From this result, we doubted '''are there one or more another PstI resutriction enzyme site except BioBrick suffix'''? | |
| - | + | ||
| - | ===PCR=== | + | ====PCR==== |
| - | + | PCR to confirm what DNA fragment is ligated to vector as insert. | |
| - | PCR to confirm what DNA fragment is | + | |
We used PCR for pT7-RBS on pSB1C3. So the result would be 80~90bp band. | We used PCR for pT7-RBS on pSB1C3. So the result would be 80~90bp band. | ||
| - | |||
{|class="hokkaidou-table-pcr-reagent" | {|class="hokkaidou-table-pcr-reagent" | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |1 ul |
|- | |- | ||
|KOD-Plus-NEO(Taq polymerase) | |KOD-Plus-NEO(Taq polymerase) | ||
| - | | | + | |1 ul |
|- | |- | ||
|dNTP | |dNTP | ||
| - | | | + | |5 ul |
|- | |- | ||
|MgSO4 | |MgSO4 | ||
| - | | | + | |3 ul |
|- | |- | ||
|KOD-Plus-NEO Buffer | |KOD-Plus-NEO Buffer | ||
| - | | | + | |5 ul |
|- | |- | ||
|Forward Primer(Biobrick prefix forward primer) | |Forward Primer(Biobrick prefix forward primer) | ||
| - | | | + | |1 ul |
|- | |- | ||
|Reverse Primer(Biobrick suffix Reverse primer) | |Reverse Primer(Biobrick suffix Reverse primer) | ||
| - | | | + | |1 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |33 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |50 ul |
|} | |} | ||
| Line 637: | Line 587: | ||
[[image:HokkaidoU2012 120718 pT7-RBS on pSB1C3 EX-F PS-R PCR.jpg|thumb|PCR product]] | [[image:HokkaidoU2012 120718 pT7-RBS on pSB1C3 EX-F PS-R PCR.jpg|thumb|PCR product]] | ||
| - | |||
| - | ===Liquid Culture=== | + | ====Liquid Culture==== |
| - | + | Liquid culture for single colony isolated(19hrs incubated) Ag43-dT on pSB1T3. | |
| - | Liquid culture for single colony isolated(19hrs | + | #Prepared 2 ml LB. |
| - | + | ||
| - | #Prepared | + | |
#Added Tet. | #Added Tet. | ||
#Resuspended one colony. | #Resuspended one colony. | ||
| - | # | + | #Incuvated 16 hrs. |
| - | + | ||
| - | + | We decided to start another ligation plan. First we digestion Ag43(K346007) and dT(B0015) with EcoRI&SpeI and EcoRI&XbaI then ligate. Ligation product cut with XbaI&SpeI and HindiIII. HindiIII can cut the site which is exist in pSB1AK3 then we can confirm Ag43-dT(about 3000bp) and pSB1K3(3000bp: cutting fragment will be about 1000bp). Next we digest pT7-RBS on pSB1C3 with SpeI only and ligate X-Ag43-dT-S and S-pSB1C3-pT7-RBS-S. If that go well, we can get pT7-RBS-Ag43-dT on pSB1C3 plasmid DNA which has never been digested with PstI. | |
| - | We decided to start another ligation plan. First we digestion Ag43(K346007) and dT(B0015) with EcoRI&SpeI and EcoRI&XbaI then ligate. Ligation product cut with XbaI&SpeI and HindiIII. HindiIII can cut the site which is exist in pSB1AK3 then we can confirm Ag43-dT(about 3000bp) and pSB1K3(3000bp: cutting fragment will be about 1000bp). Next we digest pT7-RBS on pSB1C3 with SpeI only and | + | |
| - | + | ====Liquid culture==== | |
| - | ===Liquid culture=== | + | |
| - | + | ||
Liquid culture for Ag43(K346007) | Liquid culture for Ag43(K346007) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| + | <br style="line-height: 0; clear: both;" /> | ||
</div></div> | </div></div> | ||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==July 19th== | + | ===July 19th=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | == | + | ===Plasmid extraction=== |
| - | + | Plasmid extraction for Ag43-dT on pSB1T3 and Ag43(K346007) which is inserted in pSB1C3. | |
| - | + | We used FastGene Plasmid Mini Kit(Nippon Genetics) and got 50 ul of DNA solutions. | |
| - | We used FastGene Plasmid Mini Kit(Nippon Genetics) and got | + | |
| - | + | ||
| - | ==Electrophoresis== | + | ===Electrophoresis=== |
| - | + | Electrophoresis for Ag43-dT on pSB1T3[about 5400bp] and Ag43(K346007)[about 5200bp] plasmid extraction products. | |
| - | Electrophoresis for Ag43-dT on pSB1T3[about 5400bp] and Ag43(K346007)[about 5200bp] | + | [[image:HokkaidoU2012 120719 Ag43-dT on pSB1T3 and Ag43(K346007) mini-prep product.jpg|thumb|plasmid extraction result]] |
| - | + | From this plasmid extraction result, Ag43-dT on pSB1T3 showed obviously higher DNA band than we estimated. It means '''Ag43-dT and pSB1T3 were misligated.''' And Ag43(K346007), we thought '''Ag43(K346007) DNA band existed in correct area''' because this DNA has about 5200bp and plasmid DNA migrates more far than linear DNA. Additionally, one weak 10kbp band existed in Ag43(K346007) lane. | |
| - | [[image:HokkaidoU2012 120719 Ag43-dT on pSB1T3 and Ag43(K346007) mini-prep product.jpg|thumb| | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | ===Digestion=== | ||
We conducted two digestion. | We conducted two digestion. | ||
*Digestion for Ag43(K346007) cut with EcoRI and SpeI. This DNA is for confirmation of PstI restriction enzyme cutting site. | *Digestion for Ag43(K346007) cut with EcoRI and SpeI. This DNA is for confirmation of PstI restriction enzyme cutting site. | ||
*Digestion for Ag43(K346007) and dT(B0015) cut with EcoRI & SpeI and EcoRI & XbaI. | *Digestion for Ag43(K346007) and dT(B0015) cut with EcoRI & SpeI and EcoRI & XbaI. | ||
But we cut dT PCR product which didn't have plasmid vector so we retry digestion of dT after. | But we cut dT PCR product which didn't have plasmid vector so we retry digestion of dT after. | ||
| - | + | <br /><br /> | |
| - | + | ||
Insert Ag43(K346007)(E&S) | Insert Ag43(K346007)(E&S) | ||
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |16 ul |
|- | |- | ||
|EcoRI | |EcoRI | ||
| - | | | + | |1 ul |
|- | |- | ||
|SpeI | |SpeI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |2 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |20 ul |
|} | |} | ||
| + | |||
Vector dT(E&X) | Vector dT(E&X) | ||
| Line 725: | Line 646: | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |4 ul |
|- | |- | ||
|EcoRI | |EcoRI | ||
| - | | | + | |1 ul |
|- | |- | ||
|XbaI | |XbaI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xM buffer | |10xM buffer | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |12 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |20 ul |
|} | |} | ||
| Line 748: | Line 669: | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |4 ul |
|- | |- | ||
|EcoRI | |EcoRI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |13 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |20 ul |
|} | |} | ||
| - | |||
dT(XbaI) | dT(XbaI) | ||
| Line 769: | Line 689: | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |4 ul |
|- | |- | ||
|XbaI | |XbaI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xM buffer | |10xM buffer | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |13 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |20 ul |
|} | |} | ||
| Line 790: | Line 710: | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |10 ul |
|- | |- | ||
|EcoRI | |EcoRI | ||
| - | | | + | |1 ul |
|- | |- | ||
|SpeI | |SpeI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | |7. | + | |7.8 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |20 ul |
|} | |} | ||
| Line 811: | Line 731: | ||
[[image:HokkaidoU2012 120719 Ag43 cut with E&S(each enzyme 0.1ul) and Ag43 cut with E&S(each enzyme 1ul).jpg|thumb|Digestion result]] | [[image:HokkaidoU2012 120719 Ag43 cut with E&S(each enzyme 0.1ul) and Ag43 cut with E&S(each enzyme 1ul).jpg|thumb|Digestion result]] | ||
| - | + | From this result, we confirmed Ag43(K346007) was successfully digested into two fragments and low concentration of restriction enzyme cause unperfectly cut of plasmid extraction product. But there are correct two bands in Ag43 cutting result so restriction enzyme worked. And about dT digestion, XbaI worked in halfway. We thought this is because we didn't added BSA buffer into dT digestion mix. | |
| + | And we retried digestion of dT which was plasmid extraction and gel extracted products. Recipe was same as above(E&X). | ||
| - | + | ===Gel extraction=== | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | ==Gel extraction== | + | |
| - | + | ||
Gel extraction for Ag43(K346007) digestion products.We used FastGene Gel&PCR extraction kit(NipponGenetics)and | Gel extraction for Ag43(K346007) digestion products.We used FastGene Gel&PCR extraction kit(NipponGenetics)and | ||
| - | got | + | got 50 ul of DNA solution. |
| - | + | ||
| - | + | ||
| + | <br style="line-height: 0; clear: both;" /> | ||
</div></div> | </div></div> | ||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==July 20th== | + | ===July 20th=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | ==Electrophoresis== | + | ====Electrophoresis==== |
| - | + | Electrophoresis for digestion result done yesterday(dT cut with EcoRI and XbaI). | |
| - | Electrophoresis for digestion result done | + | Added 5 ul of EtBr and Pre-migrated for 30 min. |
| - | Added | + | |
Additionaly, to confirm the concentration of Ag43(K346007) gel extract result. | Additionaly, to confirm the concentration of Ag43(K346007) gel extract result. | ||
| - | |||
[[image:HokkaidoU2012 120720 dTd- dTd+ Ag43(cut with E&S)gelextract.jpg|thumb|Digestion result]] | [[image:HokkaidoU2012 120720 dTd- dTd+ Ag43(cut with E&S)gelextract.jpg|thumb|Digestion result]] | ||
| + | From this result, we confirmed dT was successfully cut with EcoRI & SpeI. | ||
| - | + | ====Gel extraction==== | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | ==Gel extraction== | + | |
| - | + | ||
Gel extraction for Ag43(K346007) digestion products.We used FastGene Gel&PCR extraction kit(NipponGenetics)and | Gel extraction for Ag43(K346007) digestion products.We used FastGene Gel&PCR extraction kit(NipponGenetics)and | ||
| - | got | + | got 50 ul of DNA solution. |
| - | + | ||
| - | ==Ethanol precipitation== | + | ====Ethanol precipitation==== |
| - | + | ||
Ethanol precipitation for gel extraction products above. | Ethanol precipitation for gel extraction products above. | ||
| - | #Added | + | #Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. |
| - | #Centrifuged | + | #Centrifuged at 15000 rpm, 10 min at 4C. |
| - | # | + | #Removed supernatant and added 220 ul of 70% ethanol. |
| - | #Centrifuged | + | #Centrifuged at 15000 rpm, 5 min at 4C. |
| - | # | + | #Removed supernatant and dried out at room temperature after that added 5 ul of DW. |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | ====Ligation==== | ||
| + | We ligated Ag43(purified at 7/17 and 7/20) as an insert and dT as vector. | ||
| + | <br /><br /> | ||
Ag43(7/20) + dT | Ag43(7/20) + dT | ||
{|class="hokkaidou-table-ligation" | {|class="hokkaidou-table-ligation" | ||
|- | |- | ||
|Ag43 | |Ag43 | ||
| - | | | + | |2 ul |
|- | |- | ||
|dT | |dT | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |1 ul |
|- | |- | ||
|Ligation Mighty Mix(TAKARA) | |Ligation Mighty Mix(TAKARA) | ||
| - | | | + | |5 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |10 ul |
|} | |} | ||
| - | |||
| Line 889: | Line 791: | ||
|- | |- | ||
|Ag43 | |Ag43 | ||
| - | | | + | |4 ul |
|- | |- | ||
|dT | |dT | ||
| - | | | + | |2 ul |
|- | |- | ||
|Ligation Mighty Mix(TAKARA) | |Ligation Mighty Mix(TAKARA) | ||
| - | | | + | |6 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |12 ul |
|} | |} | ||
| Line 919: | Line 821: | ||
|} | |} | ||
| + | <br style="line-height: 0; clear: both;" /> | ||
| + | </div></div> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==July | + | ===July 21st=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | ==Electrophoresis== | + | ====Electrophoresis==== |
| - | + | ||
Electrophoresis for digestion and ligation products yesterday. | Electrophoresis for digestion and ligation products yesterday. | ||
| - | |||
[[image:HokkaidoU2012 120721 ag43-d+ dt-d+ ligation print.jpg|thumb|Digestion and Ligation results]] | [[image:HokkaidoU2012 120721 ag43-d+ dt-d+ ligation print.jpg|thumb|Digestion and Ligation results]] | ||
| - | There are | + | There are three bands in ligation products(Ag43(7/20) + dT(on pSB1AK3))). Lower band would be digestion result which couldn't be ligated with dT. Middle band would be successfully ligaed DNA which have about 6k bp(Ag43 has 3.1k bp and dT on pSB1AK3 has 3.2k bp). And higher band would make something dimer, we thought. |
| - | + | ====Single colony isolation==== | |
| + | Single colony isolation for incubated colonies spread yesterday. | ||
| - | == | + | ====Ethanol precipitation==== |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | ==Ethanol precipitation== | + | |
| - | + | ||
Ethanol precipitation for gel extracted Ag43(E&S) gel extraction product for digestion of PstI Star activity confirmation. | Ethanol precipitation for gel extracted Ag43(E&S) gel extraction product for digestion of PstI Star activity confirmation. | ||
| - | #Added | + | #Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. |
| - | #Centrifuged | + | #Centrifuged at 15000 rpm, 10 min at 4C. |
| - | # | + | #Removed supernatant and added 220ul of 70% ethanol. |
| - | #Centrifuged | + | #Centrifuged at 15000 rpm, 10 min at 4C. |
| - | # | + | #Removed supernatant and dried out at room temperature after that added 10 ul of DW. |
| - | + | ||
| - | ==Electrophoresis== | + | ====Electrophoresis==== |
| - | + | ||
Electrophoresis to confirm the concentration of DNA solution. | Electrophoresis to confirm the concentration of DNA solution. | ||
[[image:HokkaidoU2012 120721 Ag43(cut with E&S) Ethanol precipitation for PstItest.jpg|thumb|Ethanol precipitation result]] | [[image:HokkaidoU2012 120721 Ag43(cut with E&S) Ethanol precipitation for PstItest.jpg|thumb|Ethanol precipitation result]] | ||
| - | + | ====Digestion==== | |
| - | + | ||
| - | ==Digestion== | + | |
| - | + | ||
Digestion to confirm there are some PstI cutting site in Ag43(K346007)(cut with EcoRI and SpeI to remove PstI this parts potentially has). | Digestion to confirm there are some PstI cutting site in Ag43(K346007)(cut with EcoRI and SpeI to remove PstI this parts potentially has). | ||
| Line 985: | Line 872: | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |2 ul |
|- | |- | ||
|PstI | |PstI | ||
| - | |0. | + | |0.1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |1 ul |
|- | |- | ||
|DW | |DW | ||
| - | |6. | + | |6.9 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |10 ul |
|} | |} | ||
| - | PstI = 0. | + | PstI = 0.2 ul |
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |2 ul |
|- | |- | ||
|PstI | |PstI | ||
| - | |0. | + | |0.2 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |1 ul |
|- | |- | ||
|DW | |DW | ||
| - | |6. | + | |6.8 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |10 ul |
|} | |} | ||
| - | PstI = 0. | + | PstI = 0.5 ul |
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |2 ul |
|- | |- | ||
|PstI | |PstI | ||
| - | |0. | + | |0.5 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |1 ul |
|- | |- | ||
|DW | |DW | ||
| - | |6. | + | |6.5 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |10 ul |
|} | |} | ||
| - | PstI = 1. | + | PstI = 1.0 ul |
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |2 ul |
|- | |- | ||
|PstI | |PstI | ||
| - | | | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| - | | | + | |1 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |6 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |10 ul |
|} | |} | ||
| + | [[image:HokkaidoU2012 120721 Ag43(cut with E&S) cut with PstI(0.1, 0.2, 0.5, 1.jpg|thumb|Digestion result]] | ||
| + | From this digestion, we confirmed there is at least one PstI cutting site in Ag43 fragment because these digestion results showed same bp and same number of cutting band. | ||
| - | + | ====PCR==== | |
| - | + | PCR to confirm how ligated Ag43 fragment(s) with dT on pSB1AK3. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | ==PCR== | + | |
| - | + | ||
| - | PCR to confirm | + | |
We used Ag43 last 700bp area as primer which had designed as sequencing primer. '''If three (two forward and one reverse )Ag43 were inserted, this primer can anneal to forward Ag43 as forward primer and reverse Ag43 as reverse primer then amplify about 766bp.''' If there are no reverse Ag43, DNA can't be amplified. | We used Ag43 last 700bp area as primer which had designed as sequencing primer. '''If three (two forward and one reverse )Ag43 were inserted, this primer can anneal to forward Ag43 as forward primer and reverse Ag43 as reverse primer then amplify about 766bp.''' If there are no reverse Ag43, DNA can't be amplified. | ||
| - | + | <br /><br /> | |
PCR recipe | PCR recipe | ||
| Line 1,076: | Line 959: | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |1 ul |
|- | |- | ||
|KOD-Plus-NEO(Taq polymerase) | |KOD-Plus-NEO(Taq polymerase) | ||
| - | | | + | |1 ul |
|- | |- | ||
|dNTP | |dNTP | ||
| - | | | + | |5 ul |
|- | |- | ||
|MgSO4 | |MgSO4 | ||
| - | | | + | |3 ul |
|- | |- | ||
|KOD-Plus-NEO Buffer | |KOD-Plus-NEO Buffer | ||
| - | | | + | |5 ul |
|- | |- | ||
|Ag43-forward primer | |Ag43-forward primer | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |33 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |50 ul |
|} | |} | ||
| Line 1,125: | Line 1,008: | ||
Cycle:2~4 x 45 | Cycle:2~4 x 45 | ||
| - | |||
We failed amplification. | We failed amplification. | ||
Retry! | Retry! | ||
| - | ==PCR== | + | ====PCR==== |
| - | + | ||
We did PCR written above once again. Change some point of reaction. | We did PCR written above once again. Change some point of reaction. | ||
| - | We amplified Ag43(K346007) original DNA also as a control. | + | We amplified Ag43(K346007) original DNA also as a control.<br /> |
PCR recipe | PCR recipe | ||
| Line 1,139: | Line 1,020: | ||
|- | |- | ||
|DNA solution | |DNA solution | ||
| - | | | + | |2 ul |
|- | |- | ||
|KOD-Plus-NEO(Taq polymerase) | |KOD-Plus-NEO(Taq polymerase) | ||
| - | | | + | |1 ul |
|- | |- | ||
|dNTP | |dNTP | ||
| - | | | + | |5 ul |
|- | |- | ||
|MgSO4 | |MgSO4 | ||
| - | | | + | |3 ul |
|- | |- | ||
|KOD-Plus-NEO Buffer | |KOD-Plus-NEO Buffer | ||
| - | | | + | |5 ul |
|- | |- | ||
|Ag43-forward primer | |Ag43-forward primer | ||
| - | | | + | |2 ul |
|- | |- | ||
|DW | |DW | ||
| - | | | + | |33 ul |
|- | |- | ||
|Total | |Total | ||
| - | | | + | |50 ul |
|} | |} | ||
| Line 1,191: | Line 1,072: | ||
|} | |} | ||
Cycle:2~4 x 45 | Cycle:2~4 x 45 | ||
| - | |||
| - | |||
| - | |||
| + | <br style="line-height: 0; clear: both;" /> | ||
</div></div> | </div></div> | ||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==July | + | ===July 22nd=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | + | ====Electrophoresis==== | |
| - | ==Electrophoresis== | + | Results of digestion at 21st.<br /> |
| - | + | We expected to appear the band of about 700bp. | |
| - | Results of digestion | + | |
| - | We expected appear the band of about 700bp. | + | |
So, we use 1% and 2% agarose gel. | So, we use 1% and 2% agarose gel. | ||
[[image:HokkaidoU_2012_120722_ag43_pcr-1%_print-1.jpg|thumb|PCR result image]] | [[image:HokkaidoU_2012_120722_ag43_pcr-1%_print-1.jpg|thumb|PCR result image]] | ||
[[image:HokkaidoU2012_120722_ag43_pcr-2%_print.jpg|thumb|PCR result image]] | [[image:HokkaidoU2012_120722_ag43_pcr-2%_print.jpg|thumb|PCR result image]] | ||
| - | |||
<br /> | <br /> | ||
We can't find some band in 700~800bp... | We can't find some band in 700~800bp... | ||
| - | |||
| - | ==Liquid Culture== | + | ====Liquid Culture==== |
| - | + | ||
Liquid culture for single colony isolated Ag43-dT on pSB1AT3. | Liquid culture for single colony isolated Ag43-dT on pSB1AT3. | ||
| + | #Picked up one colony from single colony isolated platesãby platinum loop. | ||
| + | #Dipped into 2 ml of LBA. | ||
| + | #Incubated for 16 hrs. | ||
| - | + | ====Digestion==== | |
| - | + | Digested Ag43(K346oo7) plasmid extraction product with EcoRI and SpeI. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | {|class="hokkaidou-table-digestion" | ||
| + | |- | ||
| + | |DNA solution | ||
| + | |5 ul | ||
| + | |- | ||
| + | |EcoRI | ||
| + | |1 ul | ||
| + | |- | ||
| + | |SpeI | ||
| + | |1 ul | ||
| + | |- | ||
| + | |10xH buffer | ||
| + | |2 ul | ||
| + | |- | ||
| + | |DW | ||
| + | |11 ul | ||
| + | |- | ||
| + | |Total | ||
| + | |20 ul | ||
| + | |} | ||
| + | <br style="line-height: 0; clear: both;" /> | ||
| + | </div></div> | ||
<!-- DO NOT EDIT UNDER THIS LINE @iTakeshi --> | <!-- DO NOT EDIT UNDER THIS LINE @iTakeshi --> | ||
Latest revision as of 03:58, 27 September 2012
Contents |
July 16th
Ag43, dT
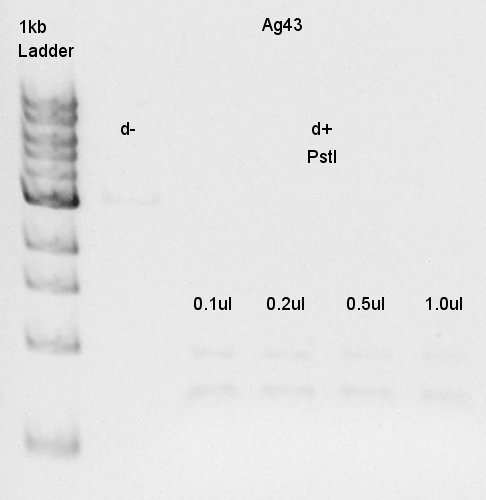
Electrophoresis
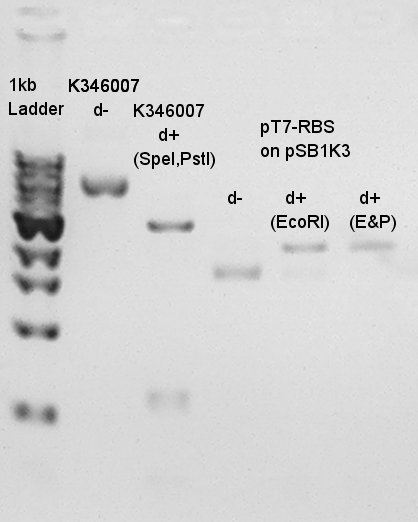
Results of digestion at 15th.
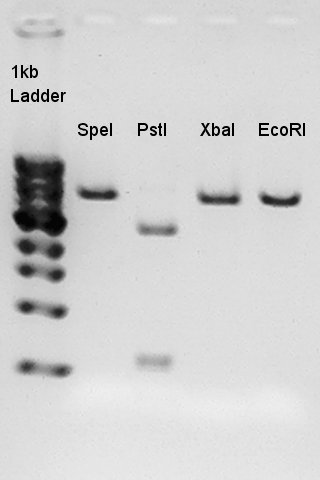
Product:Ag43(K346007)=3120bp and 2070bp
pT7-RBS on pSB1K3=2247bp
We confirmed there are some kinds of restriction enzyme site in K346007 (digested with SpeI, PstI), and pT7-RBS on pSB1K3 was successfully digested with EcoRI and PstI.ãBalance between d+(EcoRI) and d+(E&P:cut with EcoRI and PstI) is about 80bp.
Gel extraction
Gel extraction for digestion products. Used FastGene Gel&PCR extraction kit(NipponGenetics) andãgot 50 ul of DNA solution.
Ethanol precipitation
Ethanol precipitation for digested and extracted products.
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged at 15000 rpm, 10 min at 4C.
- Removed supernatant and added 220 ul of 70% ethanol.
- Centrifuged at 15000 rpm, 10 min at 4C.
- Removed supernatant and air drying at room temperature then added 5 ul of DW.
dT(B0015) would be amplifiedãincorrectly and we couldn't get enough digestion product. So we tried another DNA amplification method: PCR then digested.
PCR
PCR for dT(B0015)
| DNA solution | 1 ul |
| KOD-Plus-NEO(Taq polymerase) | 1 ul |
| dNTP | 5 ul |
| MgSO4 | 3 ul |
| KOD-Plus-NEO Buffer | 5 ul |
| Forward Primer(100bp_up forward primer) | 1 ul |
| Reverse Primer(200bp_down Reverse primer) | 1 ul |
| DW | 33 ul |
| Total | 50 ul |
| Number | Degree | Second |
| 1 | 94 | 120 |
| 2 | 98 | 10 |
| 3 | 58 | 30 |
| 4 | 68 | 30 |
| 5 | 4 | HOLD |
Cycle:2~4 x 45
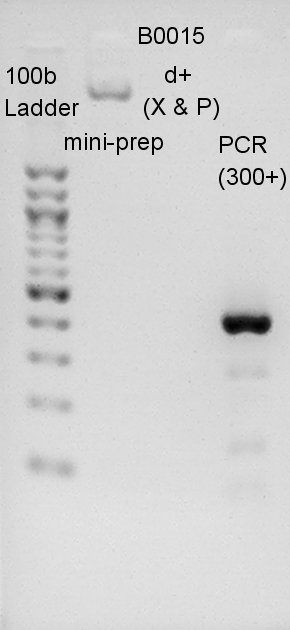
We migrated B0015 of plasmid extraction product, digestion product, and PCR product. dT done PCR would have 429bp(100bp(added by forward primer) + 129bp(dT) + 200bp(added by reverse primer)). Most bright and thick band in this image has about 300~400 bp. We thought dT was amplified successfully.
Gel extraction
Gel extraction for digestion products. We used FastGene Gel&PCR extraction kit(NipponGenetics). Got 50 ul of DNA solution.
Digestion
Digestion for dT which amplified with PCR.
Digested with XbaI and PstI.
dT
| DNA solution | 5 ul |
| XbaI | 1 ul |
| PstI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 11 ul |
| Total | 20 ul |
Ag43
Digestion result of Ag43 was incorrect. We digested Ag43 once more time.
Digestion
Digestion for Ag43 with SpeI and PstI.
| Ag43 DNA solution | 9 ul |
| SpeI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 7 ul |
| Total | 20 ul |
There are same results with digestion result of recent. We thought PstI would cut different site. What is this 500bp fragment????
Gel extraction
Gel extraction for digestion products. We used FastGene Gel&PCR extraction kit(NipponGenetics). Got 50ul of DNA solution.
Liquid Culture
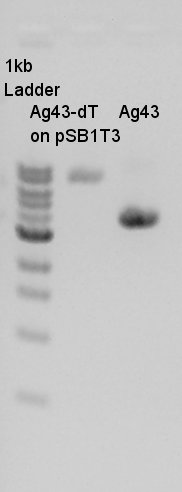
Liquid culture for single colony isolated pT7-RBS on pSB1C3 and Ag43-dT on pSB1T3.
Colonies which have Ag43-dT on pSB1T3 were closely existed so we would picked up two or more colonies.
- Picked up one (or tow?) colony from single colony isolated platesãby platinum loop.
- Dipped into 2 ml of LBC and LBT.
- Incuvated.
July 17th
Ag43-dT on pSB1T3 and pT7-RBS on pSB1C3
Plasmid extraction
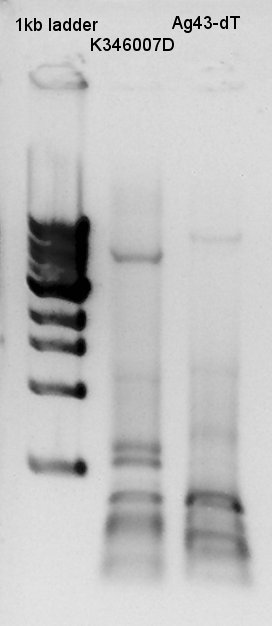
Plasmid extraction for Ag43-dT on pSB1T3 and pT7-RBS on pSB1C3 liquid culture products incuvated from yesterday(16th). We used FastGene Plasmid Mini Kit(Nippon Genetics) and got 50 ul of DNA solutions.
Electrophoresis
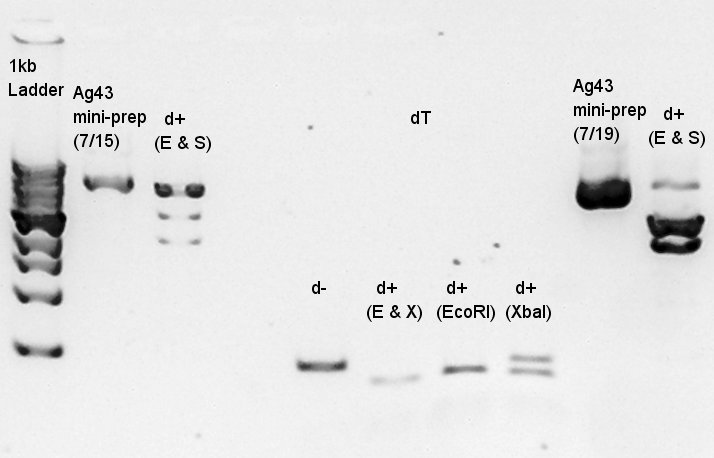
Electrophoresis for digestion product of K346007(Ag43) with EcoRI and SpeI as a reference to confirm SpeI cuts correct site or not and PstI didn't work correctly(in past experiments). And plasmid extraction products of Ag43-dT on pSB1T3 and pT7-RBS on pSB1C3 also migrated.
If digestion were succeeded, there are two bands would be seen: about 3120bp and 2070bp. And if ligation and transformation, plasmid extraction were succeeded, there would be existed two bands in each lanes: about 5000bp and 2000bp.
From this result we confirmed Ag43 was successfully digested with EcoRI and SpeI, and there were no other extra bands which had been existed double digested with EcoRI & PstI and SpeI & PstI. would PstI not work correctly?
We planed to use dT as vector and Ag43 as Insert. A problem is if dT on pSB1AK3 is used as vector and Ag43 used as insert, we can't select correct Ag43 band in gel extraction phase because DNA bp of Ag43 is nearly same as pSB1AK3. Thus we tried to use dT on pSB1T3 as vector and Ag43 on pSB1C3 as vector and ligate with standard assembly.
About plasmid extraction products, in Ag43-dT on pSB1T3, there were two plasmid DNA bands about 8000bp and 3000bp in one lane. We thought which means we failed single colony isolation then resuspended two another E.coli colonies another ligated DNA were transformed. In pT7-RBS on pSB1C3, we needed about 2000bp plasmid DNA and there were about 1500bp of DNA. Plasmid DNA migrates more far than linear DNA so we thought we got correct DNA.
To confirm plasmid extraction products were really ligated correct DNA fragments, first we extracted Ag43-dT on pSB1T3(low bp band) from TBE gel and digested with EcoRI and PstI.
dT Vector plasmid change To use dT as a vector, we needed to change plasmid backbone pAB1AK3 to pSB1T3.
Digestion
Digestion to change the plasmid backbone.
Used DNA solution as PCR product(did at 16th) and digestioned pSB1T3 were already exist.
Digestion mix
double digestion(EcoRI and PstI)
| dT PCR product | 1 ul |
| EcoRI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 15 ul |
| Total | 20 ul |
Control 1(EcoRI only)
| dT PCR product | 1 ul |
| EcoRI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 16 ul |
| Total | 20 ul |
Control 2(PstI only)
| dT PCR product | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 16 ul |
| Total | 20 ul |
We couldn't confirm digestion results.
pT7-RBS on pSB1C3 and Ag43-dT on pSB1T3
Electrophoresis and gel extraction
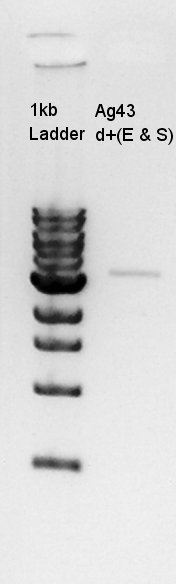
Gel extraction for Ag43-dT on pSB1T3.
We cut low bp band (see image below).
Gel Extraction for digestion products. We used FastGene Gel&PCR extraction kit(NipponGenetics).
Got 50 ul of DNA solution.
Digestion
Digestion Ag43-dT on pSB1T3 and pT7-RBS on pSB1C3 to confirm ligation was succeeded or not.
Ag43-dT on pSB1T3(30 ng/ul)
Control 1(EcoRI only)
| Ag43-dT DNA solution | 3.3 ul |
| EcoRI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 14.7 ul |
| Total | 21 ul |
Control 2(PstI only)
| Ag43-dT DNA solution | 3.3 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 14.7 ul |
| Total | 21 ul |
double digestion(EcoRI & PstI)
| Ag43-dT DNA solution | 3.3 ul |
| EcoRI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 13.7 ul |
| Total | 21 ul |
pT7-RBS on pSB1C3(40 ng/ul)
Control 1(EcoRI only)
| pT7-RBS DNA solution | 2.5 ul |
| EcoRI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 15.5 ul |
| Total | 21 ul |
Control 2(PstI only)
| pT7-RBS DNA solution | 2.5 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 15.5 ul |
| Total | 21 ul |
double digestion(EcoRI & PstI)
| pT7-RBS DNA solution | 2.5 ul |
| EcoRI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 14.5 ul |
| Total | 21 ul |
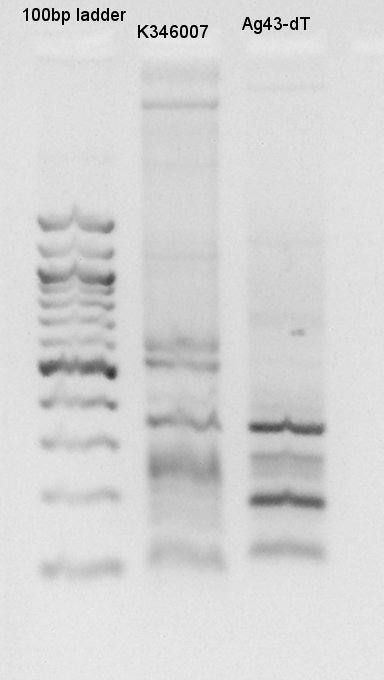
From this digestion results,
- Cut with EcoRI,PstI and E&P(EcoRI and PstI) showed different base pair bands compared with d-(digestion minus).
- Cut with E&P showed two DNA bands: about 2000bp and 500~1000 bp.
- All of digestion products existed lower place than correct band.Correct DNA would show about 5000bp(EcoRI and PstI), 3000bp and 2000bp (E&P).
July 18th
We decided change plasmid backbone of dT pSB1AK3 to pSB1T3.
If we cut Ag43-dT on pSB1AK3 with EcoRI and PstI to confirm insert DNA, DNA fragment base pair resemble each other(about 3000bp), so then we can't identify them.
digestion
Digestion to confirm what kind of restriction enzyme sites Ag43(K346007) has.
EcoRI
| DNA solution | 1 ul |
| EcoRI | 1 ul |
| 10xH buffer | 1 ul |
| DW | 7 ul |
| Total | 10 ul |
XbaI
| DNA solution | 1 ul |
| XbaI | 1 ul |
| 10xM buffer | 1 ul |
| BSA | 1 ul |
| DW | 6 ul |
| Total | 10 ul |
SpeI
| DNA solution | 1 ul |
| SpeI | 1 ul |
| 10xH buffer | 1 ul |
| DW | 7 ul |
| Total | 10 ul |
PstI
| DNA solution | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 1 ul |
| DW | 7 ul |
| Total | 10 ul |
From this result, we doubted are there one or more another PstI resutriction enzyme site except BioBrick suffix?
PCR
PCR to confirm what DNA fragment is ligated to vector as insert. We used PCR for pT7-RBS on pSB1C3. So the result would be 80~90bp band.
| DNA solution | 1 ul |
| KOD-Plus-NEO(Taq polymerase) | 1 ul |
| dNTP | 5 ul |
| MgSO4 | 3 ul |
| KOD-Plus-NEO Buffer | 5 ul |
| Forward Primer(Biobrick prefix forward primer) | 1 ul |
| Reverse Primer(Biobrick suffix Reverse primer) | 1 ul |
| DW | 33 ul |
| Total | 50 ul |
| Number | Degree | Second |
| 1 | 94 | 120 |
| 2 | 98 | 10 |
| 3 | 68 | 60 |
| 4 | 4 | HOLD |
Cycle:2~4 x 45
Liquid Culture
Liquid culture for single colony isolated(19hrs incubated) Ag43-dT on pSB1T3.
- Prepared 2 ml LB.
- Added Tet.
- Resuspended one colony.
- Incuvated 16 hrs.
We decided to start another ligation plan. First we digestion Ag43(K346007) and dT(B0015) with EcoRI&SpeI and EcoRI&XbaI then ligate. Ligation product cut with XbaI&SpeI and HindiIII. HindiIII can cut the site which is exist in pSB1AK3 then we can confirm Ag43-dT(about 3000bp) and pSB1K3(3000bp: cutting fragment will be about 1000bp). Next we digest pT7-RBS on pSB1C3 with SpeI only and ligate X-Ag43-dT-S and S-pSB1C3-pT7-RBS-S. If that go well, we can get pT7-RBS-Ag43-dT on pSB1C3 plasmid DNA which has never been digested with PstI.
Liquid culture
Liquid culture for Ag43(K346007)
July 19th
Plasmid extraction
Plasmid extraction for Ag43-dT on pSB1T3 and Ag43(K346007) which is inserted in pSB1C3. We used FastGene Plasmid Mini Kit(Nippon Genetics) and got 50 ul of DNA solutions.
Electrophoresis
Electrophoresis for Ag43-dT on pSB1T3[about 5400bp] and Ag43(K346007)[about 5200bp] plasmid extraction products.
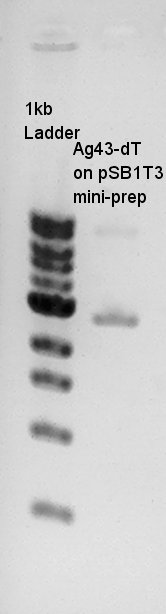
From this plasmid extraction result, Ag43-dT on pSB1T3 showed obviously higher DNA band than we estimated. It means Ag43-dT and pSB1T3 were misligated. And Ag43(K346007), we thought Ag43(K346007) DNA band existed in correct area because this DNA has about 5200bp and plasmid DNA migrates more far than linear DNA. Additionally, one weak 10kbp band existed in Ag43(K346007) lane.
Digestion
We conducted two digestion.
- Digestion for Ag43(K346007) cut with EcoRI and SpeI. This DNA is for confirmation of PstI restriction enzyme cutting site.
- Digestion for Ag43(K346007) and dT(B0015) cut with EcoRI & SpeI and EcoRI & XbaI.
But we cut dT PCR product which didn't have plasmid vector so we retry digestion of dT after.
Insert Ag43(K346007)(E&S)
| DNA solution | 16 ul |
| EcoRI | 1 ul |
| SpeI | 1 ul |
| 10xH buffer | 2 ul |
| Total | 20 ul |
Vector dT(E&X)
| DNA solution | 4 ul |
| EcoRI | 1 ul |
| XbaI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 12 ul |
| Total | 20 ul |
dT(EcoRI)
| DNA solution | 4 ul |
| EcoRI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 13 ul |
| Total | 20 ul |
dT(XbaI)
| DNA solution | 4 ul |
| XbaI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 13 ul |
| Total | 20 ul |
Ag43(K346007)(E&S)
| DNA solution | 10 ul |
| EcoRI | 1 ul |
| SpeI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 7.8 ul |
| Total | 20 ul |
From this result, we confirmed Ag43(K346007) was successfully digested into two fragments and low concentration of restriction enzyme cause unperfectly cut of plasmid extraction product. But there are correct two bands in Ag43 cutting result so restriction enzyme worked. And about dT digestion, XbaI worked in halfway. We thought this is because we didn't added BSA buffer into dT digestion mix.
And we retried digestion of dT which was plasmid extraction and gel extracted products. Recipe was same as above(E&X).
Gel extraction
Gel extraction for Ag43(K346007) digestion products.We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution.
July 20th
Electrophoresis
Electrophoresis for digestion result done yesterday(dT cut with EcoRI and XbaI). Added 5 ul of EtBr and Pre-migrated for 30 min. Additionaly, to confirm the concentration of Ag43(K346007) gel extract result.
From this result, we confirmed dT was successfully cut with EcoRI & SpeI.
Gel extraction
Gel extraction for Ag43(K346007) digestion products.We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution.
Ethanol precipitation
Ethanol precipitation for gel extraction products above.
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged at 15000 rpm, 10 min at 4C.
- Removed supernatant and added 220 ul of 70% ethanol.
- Centrifuged at 15000 rpm, 5 min at 4C.
- Removed supernatant and dried out at room temperature after that added 5 ul of DW.
Ligation
We ligated Ag43(purified at 7/17 and 7/20) as an insert and dT as vector.
Ag43(7/20) + dT
| Ag43 | 2 ul |
| dT | 2 ul |
| DW | 1 ul |
| Ligation Mighty Mix(TAKARA) | 5 ul |
| Total | 10 ul |
Ag43(7/17) + dT
| Ag43 | 4 ul |
| dT | 2 ul |
| Ligation Mighty Mix(TAKARA) | 6 ul |
| Total | 12 ul |
Ligation reaction time was written below.
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
July 21st
Electrophoresis
Electrophoresis for digestion and ligation products yesterday.
There are three bands in ligation products(Ag43(7/20) + dT(on pSB1AK3))). Lower band would be digestion result which couldn't be ligated with dT. Middle band would be successfully ligaed DNA which have about 6k bp(Ag43 has 3.1k bp and dT on pSB1AK3 has 3.2k bp). And higher band would make something dimer, we thought.
Single colony isolation
Single colony isolation for incubated colonies spread yesterday.
Ethanol precipitation
Ethanol precipitation for gel extracted Ag43(E&S) gel extraction product for digestion of PstI Star activity confirmation.
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged at 15000 rpm, 10 min at 4C.
- Removed supernatant and added 220ul of 70% ethanol.
- Centrifuged at 15000 rpm, 10 min at 4C.
- Removed supernatant and dried out at room temperature after that added 10 ul of DW.
Electrophoresis
Electrophoresis to confirm the concentration of DNA solution.
Digestion
Digestion to confirm there are some PstI cutting site in Ag43(K346007)(cut with EcoRI and SpeI to remove PstI this parts potentially has).
| Used DNA | K346007 cut with EcoRI and SpeI |
| Concentration(ng/ul) | 25 |
| Used DNA volume(ul) | 2 |
| theoretical ez value(ul) | 0.02 |
PstI = 0.1ul
| DNA solution | 2 ul |
| PstI | 0.1 ul |
| 10xH buffer | 1 ul |
| DW | 6.9 ul |
| Total | 10 ul |
PstI = 0.2 ul
| DNA solution | 2 ul |
| PstI | 0.2 ul |
| 10xH buffer | 1 ul |
| DW | 6.8 ul |
| Total | 10 ul |
PstI = 0.5 ul
| DNA solution | 2 ul |
| PstI | 0.5 ul |
| 10xH buffer | 1 ul |
| DW | 6.5 ul |
| Total | 10 ul |
PstI = 1.0 ul
| DNA solution | 2 ul |
| PstI | 1 ul |
| 10xH buffer | 1 ul |
| DW | 6 ul |
| Total | 10 ul |
From this digestion, we confirmed there is at least one PstI cutting site in Ag43 fragment because these digestion results showed same bp and same number of cutting band.
PCR
PCR to confirm how ligated Ag43 fragment(s) with dT on pSB1AK3.
We used Ag43 last 700bp area as primer which had designed as sequencing primer. If three (two forward and one reverse )Ag43 were inserted, this primer can anneal to forward Ag43 as forward primer and reverse Ag43 as reverse primer then amplify about 766bp. If there are no reverse Ag43, DNA can't be amplified.
PCR recipe
| DNA solution | 1 ul |
| KOD-Plus-NEO(Taq polymerase) | 1 ul |
| dNTP | 5 ul |
| MgSO4 | 3 ul |
| KOD-Plus-NEO Buffer | 5 ul |
| Ag43-forward primer | 2 ul |
| DW | 33 ul |
| Total | 50 ul |
| Number | Degree | Second |
| 1 | 94 | 120 |
| 2 | 98 | 10 |
| 3 | 58 | 60 |
| 4 | 4 | HOLD |
Cycle:2~4 x 45
We failed amplification.
Retry!
PCR
We did PCR written above once again. Change some point of reaction.
We amplified Ag43(K346007) original DNA also as a control.
PCR recipe
| DNA solution | 2 ul |
| KOD-Plus-NEO(Taq polymerase) | 1 ul |
| dNTP | 5 ul |
| MgSO4 | 3 ul |
| KOD-Plus-NEO Buffer | 5 ul |
| Ag43-forward primer | 2 ul |
| DW | 33 ul |
| Total | 50 ul |
| Number | Degree | Second |
| 1 | 94 | 120 |
| 2 | 98 | 10 |
| 3 | 58 | 30 |
| 4 | 68 | 30 |
| 5 | 4 | HOLD |
Cycle:2~4 x 45
July 22nd
Electrophoresis
Results of digestion at 21st.
We expected to appear the band of about 700bp.
So, we use 1% and 2% agarose gel.
We can't find some band in 700~800bp...
Liquid Culture
Liquid culture for single colony isolated Ag43-dT on pSB1AT3.
- Picked up one colony from single colony isolated platesãby platinum loop.
- Dipped into 2 ml of LBA.
- Incubated for 16 hrs.
Digestion
Digested Ag43(K346oo7) plasmid extraction product with EcoRI and SpeI.
| DNA solution | 5 ul |
| EcoRI | 1 ul |
| SpeI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 11 ul |
| Total | 20 ul |
 "
"