Team:Bielefeld-Germany/Results/substrate
From 2012.igem.org
(→Outlook) |
(→HPLC analysis of polycylic aromatic hydrocarbons) |
||
| (66 intermediate revisions not shown) | |||
| Line 14: | Line 14: | ||
== Introduction == | == Introduction == | ||
| - | + | To investigate the degradation of different substrates with laccases several experiments were performed. For the measurements the four produced bacterial laccases (BHAL, ECOL, TTHL and BPUL) were used. The reactions were measured before and after an incubation with the laccases via high performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry. The HPLC was used particularly for analysis of degradation rates after defined time points. With these results it is possible to compare the different laccases in respect to their degradation feasibilities. | |
| - | To detect degradation products of estradiol and ethinyl estradiol after laccase treatment different analysis via | + | To detect degradation products of estradiol and ethinyl estradiol after laccase treatment different analysis via LC-MS and LC-MS-MS were done. We identified two compounds for both, estradiol and ethinyl estradiol, which are probable degradation products after laccase treatment. |
=Degradation measurements with high performance liquid chromatography= | =Degradation measurements with high performance liquid chromatography= | ||
==Dilution series of different estrogens== | ==Dilution series of different estrogens== | ||
| - | At first dilution series of all different substrates were measured. It was possible measure estradiol and ethinyl estradiol | + | |
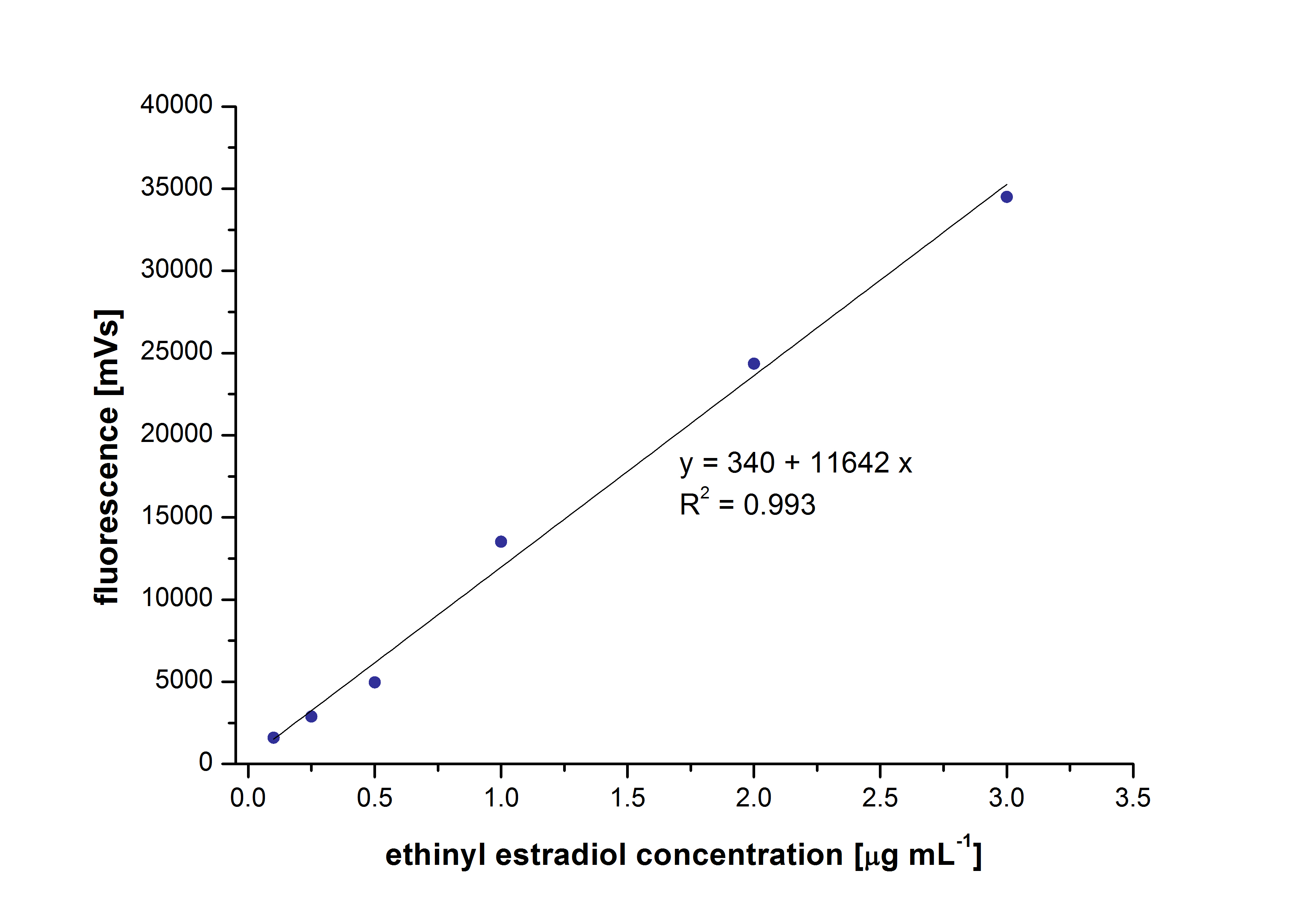
| - | The retention time for estradiol is 4.4 minutes and for ethinyl estradiol 4.9 minutes. For all estrogens the same extinction and emission values could be used: Ex <sub>230</sub>, Em<sub>310</sub>. | + | [[File:Bielefeld2012_EthinylEstradiol.jpg|400px|thumb|right|'''Figure 1:''' The calibration curve of ethinyl estradiol as an example. The concentrations were measured between 0.1 µg mL <sup>-1</sup> and 3 µg mL <sup>-1</sup>.]] |
| + | |||
| + | At first dilution series of all different substrates were measured. It was possible to measure calibration curves for estradiol and ethinyl estradiol but not for estrone. This was probably caused by its bad solubility. | ||
| + | The retention time for estradiol is 4.4 minutes and for ethinyl estradiol 4.9 minutes. For all estrogens the same extinction and emission values could be used: Ex<sub>230</sub>, Em<sub>310</sub>. | ||
<br style="clear: both" /> | <br style="clear: both" /> | ||
| - | + | ||
<br style="clear: both" /> | <br style="clear: both" /> | ||
| + | |||
==Degradation of estrogens== | ==Degradation of estrogens== | ||
| - | |||
| - | [[File:Bielefeld2012_Ohne_ABTS.png| | + | [[File:Bielefeld2012_Ohne_ABTS.png|400px|thumb|right|'''Figure 2:''' Degradation of estradiol (dark green) and ethinyl estradiol (light green) with the different laccases after 5 hours without ABTS. In the graph it is shown that the bought laccase TVEL0 which was used as positive control is able to degrade more than 90 percent of the used substrates. None of the bacterial laccases are able to degrade ethinyl estradiol without ABTS but estradiol is degraded in a range from 16 %(ECOL) to 55 % (TTHL). The original concentrations of substrates were 2 µg per approach. (n = 4)]] |
| - | |||
| - | [[File:Bielefeld2012_Mit_ABTS.png| | + | The measurements were made to test if the produced laccases were able to degrade different hormones. Therefore the produced laccases were inserted in the same concentrations (3 µg mL<sup>-1</sup>) to the different measurement approaches. To work with the correct pH value (which were measured by the Team Activity Test) Britton Robinson buffer at pH 5 was used for all measurements. The initial substrate concentration was 5 µg mL<sup>-1</sup>. The results of the reactions without ABTS are shown in Figure 2. On the Y-axis the percentages of degraded estradiol (blue) and ethinyl estradiol (red) are indicated. The X-axis displays the different tested laccases. The degradation was measured at t<sub>0</sub> and after five hours of incubation at 30 °C. The negative control was the substrate in Britton Robinson buffer and showed no degradation of the substrates. The bought laccase TVEL0 which is used as positive control is able to degrade 94.7 % estradiol and 92.7 % ethinyl estradiol. The laccase BPUL (from ''Bacillus pumilus'') degraded 35.9 % of used estradiol after five hours. ECOL was able to degrade 16.8 % estradiol. BHAL degraded 30.2 % estradiol. The best results were determined with TTHL (laccase from ''Thermus thermophilus''). Here the percentage of degradation amounted 55.4 %. |
| + | |||
| + | [[File:Bielefeld2012_Mit_ABTS.png|400px|thumb|left|'''Figure 3:''' Degradation of estradiol (blue) and ethinyl estradiol (red) with the different laccases after 10 minutes hours with ABTS added. The commercial laccase TVEL0 which was used as positive control is able to degrade all of the used substrates. The bacterial laccase BPUL degraded 100 % of ethinyl estradiol and estradiol. ECOL the laccase from ''E. coli'' degraded 6.7 % estradiol and none of the used ethinyl estradiol. BHAL degraded 46.9 % of estradiol but no ethinyl estradiol. The laccase TTHL from ''Thermus thermophilus'' degraded 29.5 % of estradiol and 9.8 % ethinyl estradiol. The original concentrations of substrates were 2 µg per approach. (n = 4)]] | ||
| + | |||
| + | The results of the reactions of the laccases with addition of ABTS are shown in Figure 3. The experimental set ups were the same as the reaction approach without ABTS described above. The X-axis displays the different tested laccases. On the Y-axis the percentages of degraded estradiol (blue) and ethinyl estradiol (red) are shown. The degradation was measured at t<sub>0</sub> and after five hours of incubation at 20 °C. The negative control showed no degradation of estradiol. 6.8 % of ethinyl estradiol was decayed. The positive control TVEL0 is able to degrade 100 % estradiol and ethinyl estradiol. The laccase BPUL (from ''Bacillus pumilus'') degraded 46.9 % of used estradiol after ten minutes incubation. ECOL was able to degrade 6.7 % estradiol. BHAL degraded 46.9 % estradiol. With TTHL (laccase from ''Thermus thermophilus'') a degradation 29.5 % were determined. | ||
| + | <br style="clear: both" /> | ||
=Spectrofluorophotometer Analysis= | =Spectrofluorophotometer Analysis= | ||
<div style="text-align:justify;"> | <div style="text-align:justify;"> | ||
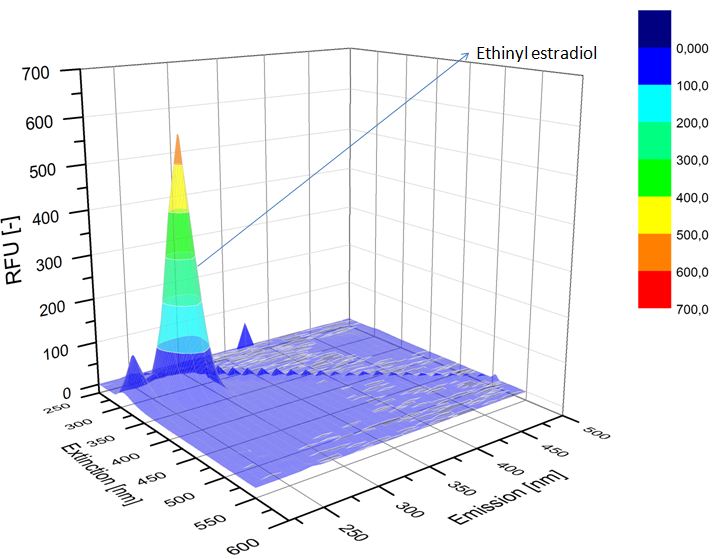
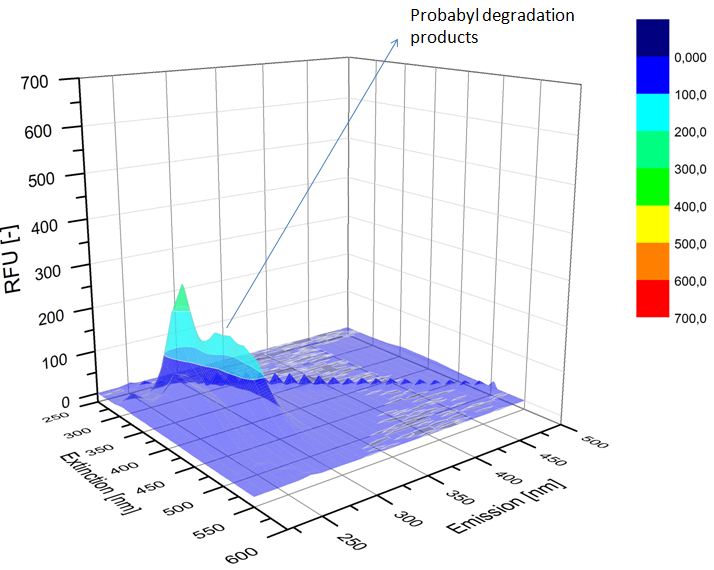
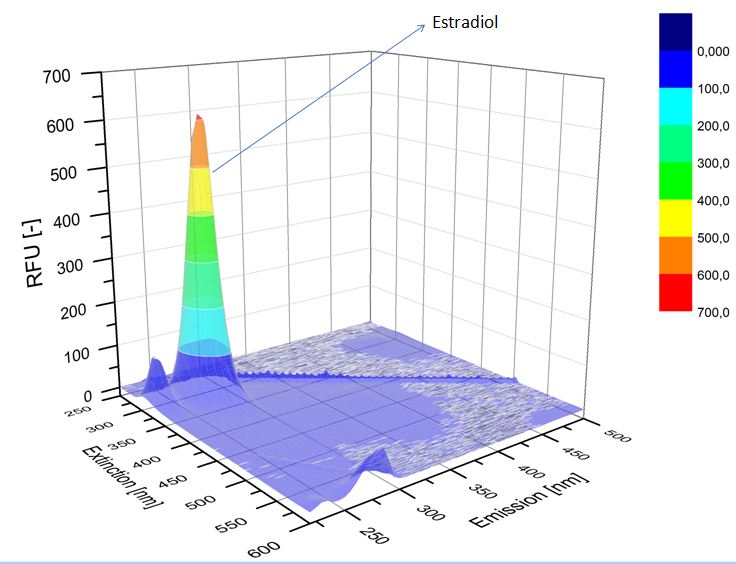
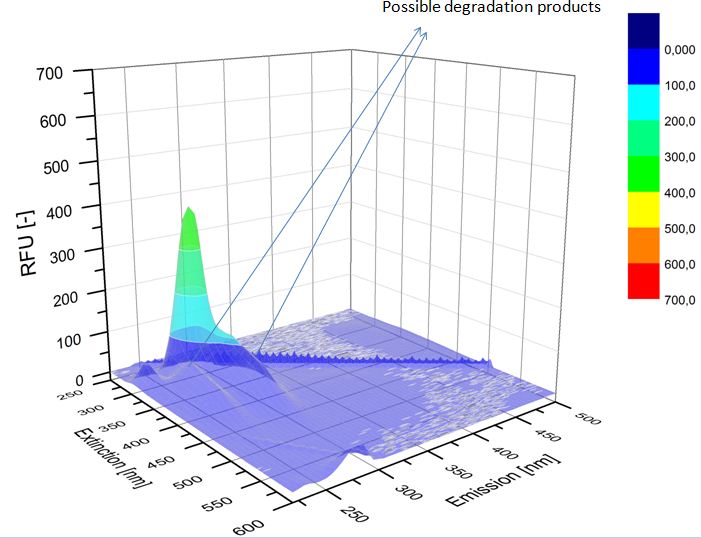
| - | We analyzed the degradation of our substrates with | + | We analyzed the degradation of our substrates with a spectrofluorophotometer. As you can see in the figures below the ethinyl estradiol and estradiol are degraded over night. Figure 4 shows the ethinyl estradiol without laccase treatment, Figure 5 shows that no more ethinyl estradiol can be detected in the sample after the degradation and new peaks appear which might represent possible degradation products. In Figure 6 you can see the estradiol control without laccases. Like ethinyl estradiol theestradiol peak is reduced after the degradation and new peaks appear indicating that those are new degradation products. |
| - | [[File:Bielefeld2012-ethinylestradiol-withoutLaccase-spectrofluorophotometer.JPG|thumb|350px|left|'''Figure | + | [[File:Bielefeld2012-ethinylestradiol-withoutLaccase-spectrofluorophotometer.JPG|thumb|350px|left|'''Figure 4:''' Ethinyl estradiol control without laccases. Ethinyl estradiol was measured in spectrofluorophotometer without laccase treatment to have a control.]] [[File:Bielefeld2012-Ethinylestradiol-verdau-spectroflurophotometer.JPG|thumb|350px|right|'''Figure 5:''' Ethinyl estradiol degradation (with TVEL0). The ethinyl estradiol peak disappeared and some new peaks, probable degradation products, occurred.]] |
<br style="clear: both" /> | <br style="clear: both" /> | ||
| - | [[File:Bielefeld2012-estradiol-control-spectroflurophotometer.JPG|thumb|350px|left|'''Figure | + | [[File:Bielefeld2012-estradiol-control-spectroflurophotometer.JPG|thumb|350px|left|'''Figure 6:''' Estradiol control without laccases.]] |
| - | [[File:Bielefeld2012-estradiol-degradation-spectroflurophotometer.JPG|thumb|350px|right|'''Figure | + | [[File:Bielefeld2012-estradiol-degradation-spectroflurophotometer.JPG|thumb|350px|right|'''Figure 7:''' Degradation (with TVEL0) of estradiol. It is shown that some estradiol is left but probable degradation products appeared.]] |
<br style="clear: both" /> | <br style="clear: both" /> | ||
=Liquid chromatography–mass spectrometry= | =Liquid chromatography–mass spectrometry= | ||
=== Dilution series === | === Dilution series === | ||
| - | Our substrates are soluble in methanol. We set the standards to a concentration of 1 mg mL<sup>-1</sup>. The | + | Our substrates are soluble in methanol. We set the standards to a concentration of 1 mg mL<sup>-1</sup>. The detection limit for the LC-MS was evaluated at a concentration of 10 µg L<sup>-1</sup> for the substrates estrone and estradiol. The same limit of detection was used for ethinyl estradiol and anthracene. We only used those four substrates. For all LC-MS sample preparations we used the ''T. versicolor'' laccases. The dilution series was prepared in methanol and 50 % acetonitril-water (v/v). |
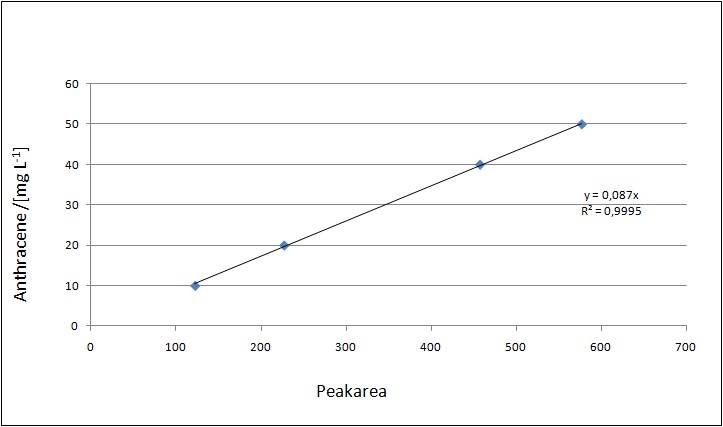
| - | [[File:Bielefeld2012-calibrationcurve-Anthracen.jpg|thumb|400px|left|'''Figure | + | [[File:Bielefeld2012-calibrationcurve-Anthracen.jpg|thumb|400px|left|'''Figure 8:''' Anthracene calibration curve. ]] |
| - | [[File:Bielefeld2012-Estrone-calibrationcurve.JPG|thumb|400px|right|'''Figure | + | [[File:Bielefeld2012-Estrone-calibrationcurve.JPG|thumb|400px|right|'''Figure 9:''' Estrone calibration curve.]] |
<br style="clear: both" /> | <br style="clear: both" /> | ||
=== Degradation results === | === Degradation results === | ||
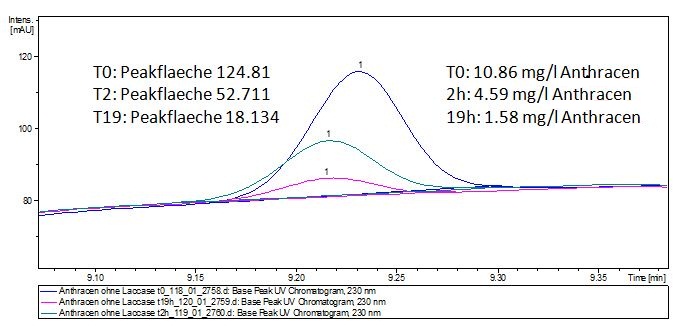
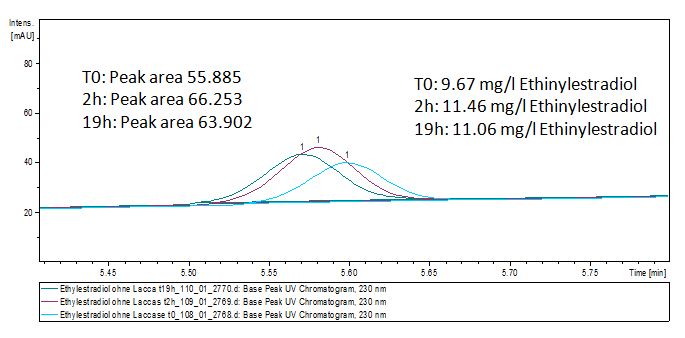
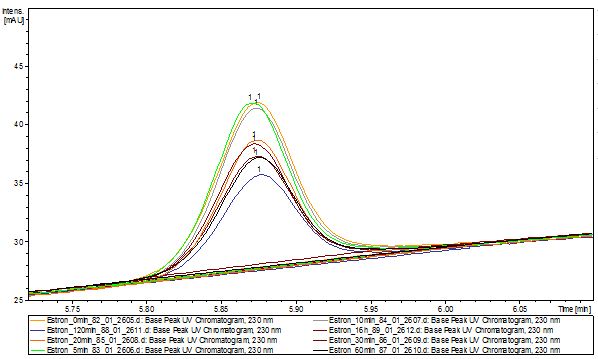
| - | The TVEL0 was able to degrade the synthetic estradiol (Fig. | + | The TVEL0 was able to degrade the synthetic estradiol (Fig. 10) and probably anthracene (Fig. 12). The ethinyl estradiol control showed that it is stable in the used media (Fig. 11). Anthracene disintegrates in the Britton Robinsoon Buffer. But it could be observed, that there is less anthracene measurable with the LC-MS. The results indicate, that the laccase is able to degrade anthracene (Fig. 13). Estrone (Fig. 14) and estradiol (Fig. 15) were degraded as well. Using estrone it could not be identify any degradation products. The reason for this could be that the products are not detectable with LC-MS or with the applied methods. Peaks in the degradation of estradiol have been shown but we were not able to identify them. It could be degradation products. In the following figures the results of the LC-MS measurements are presented. |
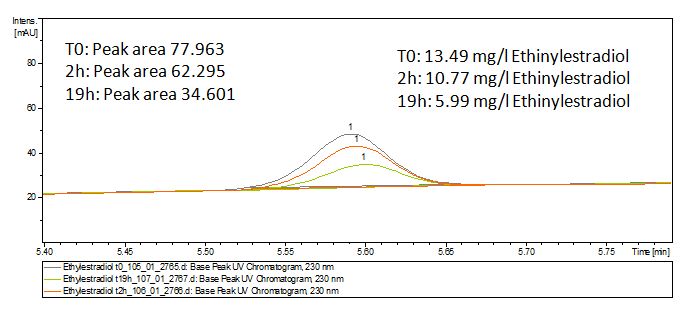
| - | [[File:Bielefeld2012-Ethinylestradiol-degradation-LCMS.JPG|thumb|left|350px|'''Figure | + | [[File:Bielefeld2012-Ethinylestradiol-degradation-LCMS.JPG|thumb|left|350px|'''Figure 10:''' Ethinyl estradiol + TVEL0 measured by LC-MS. It is shown that the over night sample has only half of the substrate left.]] |
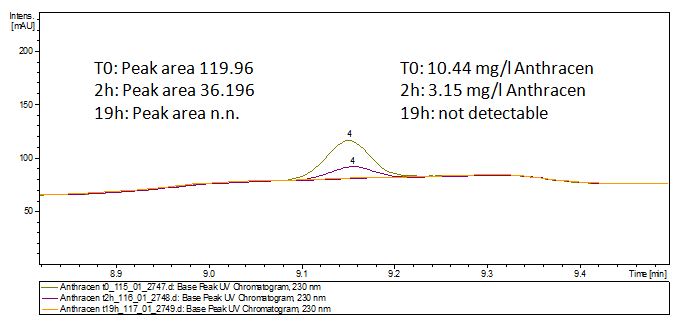
| - | [[File:Bielefeld2012-Anthracen-degradation-LCMS.JPG|thumb|right|350px|'''Figure | + | [[File:Bielefeld2012-Anthracen-degradation-LCMS.JPG|thumb|right|350px|'''Figure 12:''' Anthracene + TVEL0. In the over night sample there are no detectable amounts of anthracene left.]] <br style="clear: both" /> |
| - | [[File:Bielefeld2012-Anthracen-standart1.JPG|right|thumb|350px|'''Figure | + | [[File:Bielefeld2012-Anthracen-standart1.JPG|right|thumb|350px|'''Figure 13:''' The negative control for anthracene without laccases. It is shown the concentration of anthracene decreases. This is caused by the Britton Robinson Buffer.]] |
| - | [[File:Bielefeld2012-Ethinylestradiol-standart100.JPG|thumb|left|350px|'''Figure | + | [[File:Bielefeld2012-Ethinylestradiol-standart100.JPG|thumb|left|350px|'''Figure 11:''' Our ethinyl estradiol negative control without laccase. Variation on the peaks is probably caused by a pipetting mistake.]] <br style="clear: both" /> |
| - | [[File:Bielefeld2012-Estrone-degradation-LCMS.JPG|thumb|left|350px|'''Figure | + | [[File:Bielefeld2012-Estrone-degradation-LCMS.JPG|thumb|left|350px|'''Figure 14:''' Estrone + TVEL0. The peaks shows that estrone is degraded but after incubation over night it is still estrone left.]] |
| - | [[File:Bielefeld2012-Estradiol-degradation-LCMS.JPG|thumb|right|350px|'''Figure | + | [[File:Bielefeld2012-Estradiol-degradation-LCMS.JPG|thumb|right|350px|'''Figure 15:''' Estradiol degradation analyses with mass-spectrometry. On the X-axis the retention time is listed. The Y-axis shows the mass/charge ratio. From white to red the intensity of the measured samples is presented. On the figure above you can see the t<sub>0</sub> estradiol while the figure below shows the degradation. The analytes retended in the first minute are the media soillings. Since we know that the retention time of estradiol is on min 5 we could see that over night no more estradiol is left and some other peaks appear which are probably degradation products]] |
<br style="clear: both" /> | <br style="clear: both" /> | ||
| - | We also tried to measure the degradation using mass-spectrometry. Since quantification via mass-spectrometry is difficult regarding the ionization of the analytes, we quantified our substrates by UV-light. Nevertheless, mass spectrometry enables identification of possible degradation products. We analyzed estradiol degradation in detail (Fig. | + | We also tried to measure the degradation using mass-spectrometry. Since quantification via mass-spectrometry is difficult regarding the ionization of the analytes, we quantified our substrates by UV-light. Nevertheless, mass spectrometry enables identification of possible degradation products. We analyzed estradiol degradation in detail (Fig. 15), resulting in the detection of possible chemical compounds generated during the (enzymatic) degradation. |
<br style="clear: both" /> | <br style="clear: both" /> | ||
| - | Since we have seen some possible degradation products, we used more estradiol, ethinyl estradiol and more laccase for the reaction to the LC-MS measurement. We found out that after the laccase treatment two new peaks | + | |
| - | [[File:Bielefeld2012-Estradiol-MS-measurement.JPG|thumb|350px|left|'''Figure | + | === Further analysis (after Regionals Amsterdam) === |
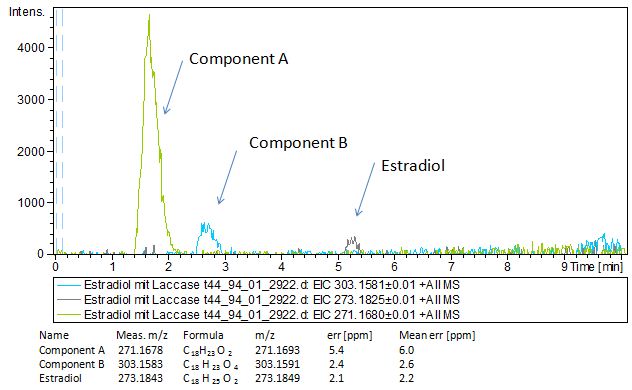
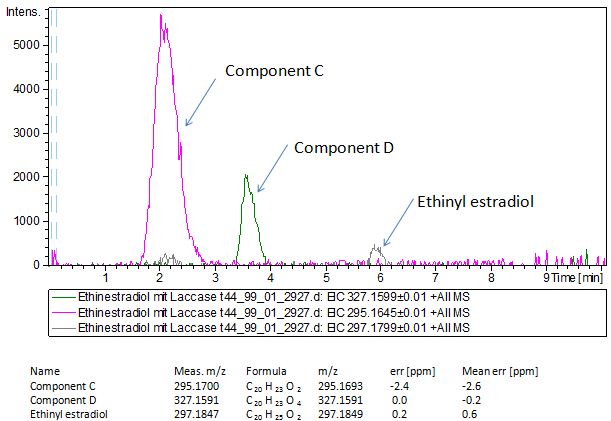
| - | [[File:Bielefeld2012-EthinylEstradiol-MS-measurement.JPG|thumb|350px|right|'''Figure | + | Since we have seen some possible degradation products, we used more estradiol, ethinyl estradiol (250µg L<sup> -1 </sup> compare to 50µg L<sup> -1 </sup>) and more laccase (0,35U compare to 0,1U) for the reaction to the LC-MS measurement. We found out that after the laccase treatment two new peaks appeared in both the estradiol and ethinyl estradiol. A check against databases could not identify those components so we did an MS-MS on component A respectivly on component C. Since component B is fewer in concentration then component A, we could not find out anything about it (Fig. 16 and Fig. 17). To note the chemical formulares are M+H values. For the real chemical formula you have to deduct this H. |
| + | [[File:Bielefeld2012-Estradiol-MS-measurement.JPG|thumb|350px|left|'''Figure 16:''' Mass spectromerty measurement of estradiol. On the X-axis you can see the Time in minutes and on the Y-axis the relative intensity. You can see two new peaks component A and B after laccase treatment. Component A was taken for MS-MS]] | ||
| + | [[File:Bielefeld2012-EthinylEstradiol-MS-measurement.JPG|thumb|350px|right|'''Figure 17:''' Mass spectromerty measurement of ethinyl estradiol. On the X-axis you can see the time in minutes and on the Y-axis the relative intensity. You can see two new peaks component C and D after laccase treatment. Component C was taken for MS-MS]] | ||
<br style="clear: both" /> | <br style="clear: both" /> | ||
| - | The | + | |
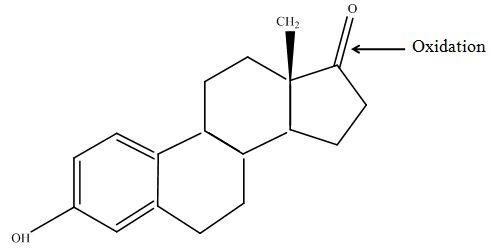
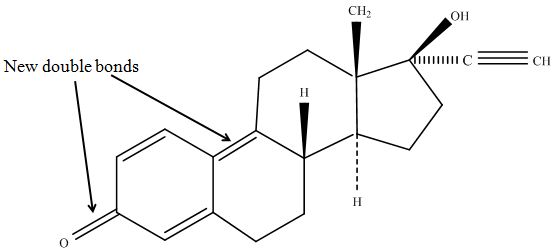
| - | [[File:Bielefeld2012-estradiol-MS-kuck-suggest.JPG|thumb|350px|left|'''Figure | + | The tandem mass spectrometry results showed two peaks in every measurement for possible degradation products. Compared to the native estradiol the component A peak embodies a molecule with two hydrogen atoms less than the native, the same does component C for ethinly estradiol. These peaks were discussed in a short chat with Prof. Dr. Dietmar Kuck, an organic chemist with a lot experience in mass spectrometry. This conversation led to two plausible models for the degradation. The first was a reaction at the hydroxy group of the five-ring of the estradiol (Fig. 18), oxidizing it to a ketone (resulting in estrone as the degradation product). This idea seemed improbable, because ethinyl estradiol cannot be oxidized at that position, but its degradation product also had two hydrogen less than the native (it is impossible to oxidize the hydroxy group of the five-ring to a ketone without losing the ethinyl group or breaking the ring (Fig. 19)). The second model was that the laccase radicalizes the hydroxy group of the phenolic group of the estradiol and ethinyl estradiol, as described in literature, building a phenoxy radical in the first step. In the second step hydrogen is split off on the tertiary carbon in para position finally leading to a quinone like structure (Figure 19). Without knowing the mechanism behind this reaction, this seems the most probable model of the degradation. |
| - | [[File:Bielefeld2012- | + | |
| + | [[File:Bielefeld2012-estradiol-MS-kuck-suggest.JPG|thumb|350px|left|'''Figure 18:''' First possible structure of the degradation product (component A) after laccase treatment. The secondary alcohol is oxidized to a ketone and the product corresponds to estrone. This structure was designed with ChemBioDraw Ultra 12.0 after discussion with Prof. Kuck on our MS/MS data.]] | ||
| + | [[File:Bielefeld2012-EE2-MSMS-suggestion-Kuck.JPG|thumb|350px|right|'''Figure 19:''' Suggested chemical structure of the oxidized ethinyl estradiol. The phenolic part of the molecule has changed in a quinone like structure. This structure was designed with ChemBioDraw Ultra 12.0 after discussion with Prof. Kuck on our MS/MS data.]] | ||
| + | <br style="clear: both" /> | ||
| + | The second peaks in the chromatograms (component B and D) embody molecules with two oxygen more and two hydrogen less than the native. We postulate that this might be a transition state where O<sub>2</sub> is radically added to the phenolic ring in otho- and meta-position. To that point, there is no data to consolidate this adoption. Component B and D were also fragmented in the tandem mass spectrometry for further analysis, but the resulting peaks could not be differed from the background noise. | ||
<br style="clear: both" /> | <br style="clear: both" /> | ||
= Further substrate analysis via HPLC = | = Further substrate analysis via HPLC = | ||
== HPLC analysis of polycylic aromatic hydrocarbons == | == HPLC analysis of polycylic aromatic hydrocarbons == | ||
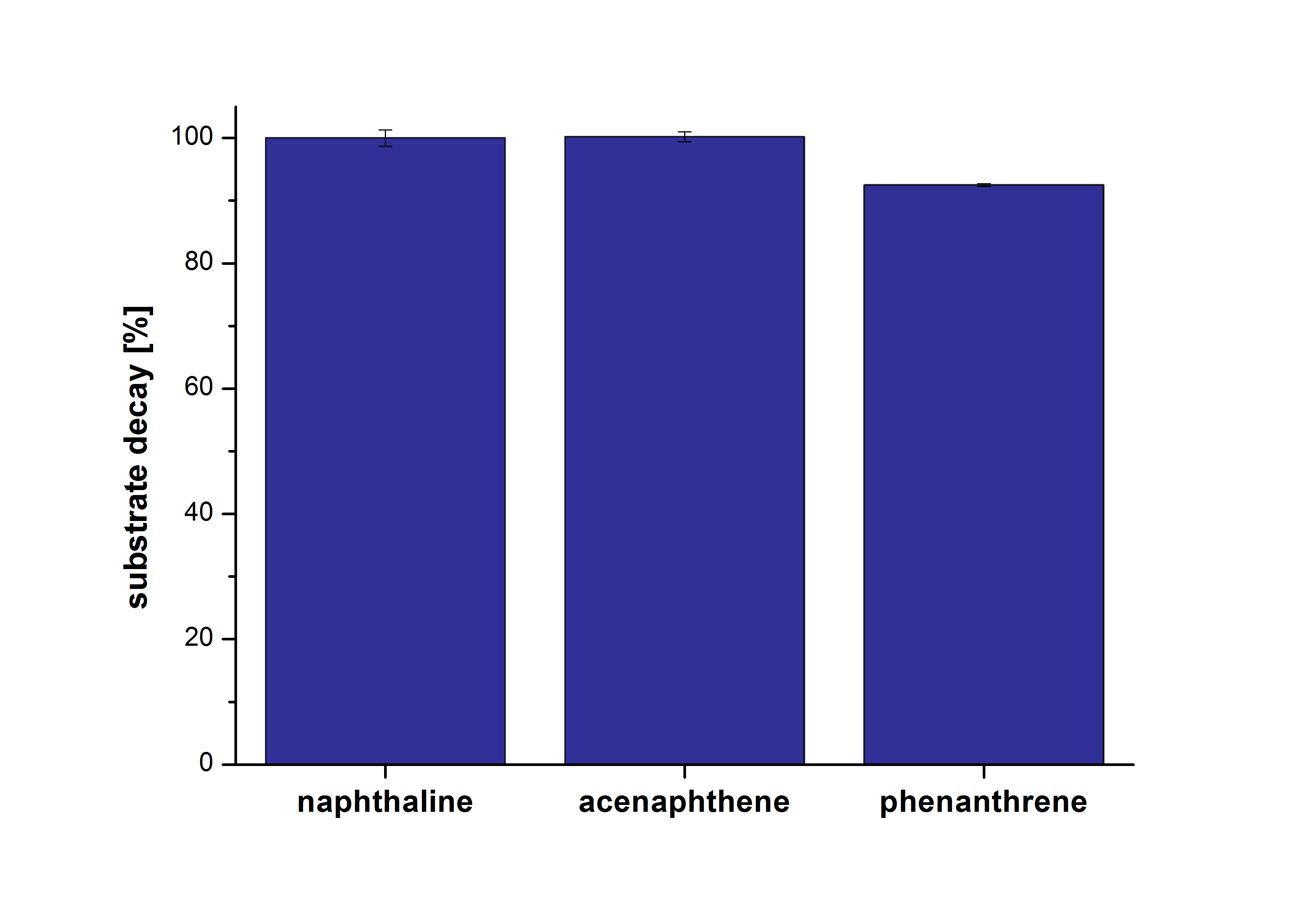
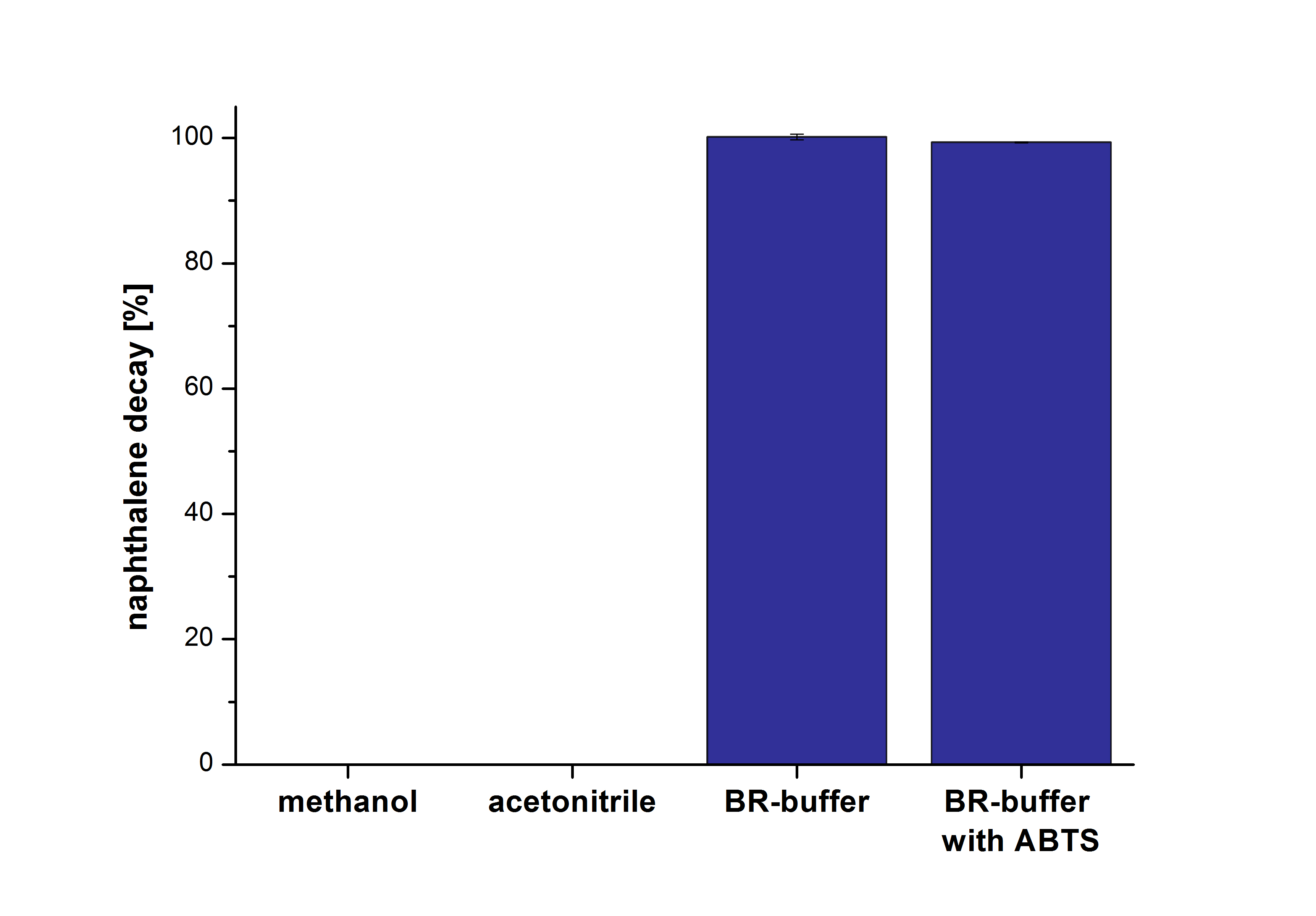
| - | The results of our negative control measurements of polycyclic aromatic hydrocarbons showed that the PAHs decayed without laccase treatment after one hour in Britton Robinson (BR)-buffer. This is shown in Figure | + | The results of our negative control measurements of polycyclic aromatic hydrocarbons showed that the PAHs decayed without laccase treatment after one hour in Britton Robinson (BR)-buffer. This is shown in Figure 20. <br>The next step was to check which substances in the reaction approach may cause the decay. Therefore naphthalene was dissolved in methanol (which is the solvent for the substrate and used for stopping the reaction), acetonitrile (which could be used as alternative solvent) and BR-buffer with and without ABTS. The results are shown in Figure 21. Using pure methanol and acetonitrile naphthalene is not decayed. In BR-buffer with and without ABTS the decay is nearly completed after one hour treatment. So BR-buffer seems a bad choice to test the degradation of naphtalene under laccase treatment. |
| - | [[File:Bielefeld2012_PAH.png|400px|thumb|left|''' Figure : Decay of the PAHs naphthalene, acenaphthene and phenantrene in BR-buffer at 30 °C after one hour. | + | [[File:Bielefeld2012_PAH.png|400px|thumb|left|''' Figure 20:''' Decay of the PAHs naphthalene, acenaphthene and phenantrene in BR-buffer at 30 °C after one hour. The initial concentration was 1 µg mL <sup>-1</sup> for all PAHs. After one hour nearly all PAHs decayed completely. (n=2)]] |
| - | [[File:Bielefeld2012_Naphthalene.png|400px|thumb|right|'''Figure : Naphthalene decay in four different approaches at 30 °C after one hour. | + | [[File:Bielefeld2012_Naphthalene.png|400px|thumb|right|'''Figure 21:''' Naphthalene decay in four different approaches at 30 °C after one hour. Dissolved in methanol, dissolved in acetonitrile, with BR-buffer and with BR-buffer together with ABTS. (n=2)]] |
<br style="clear: both" /> | <br style="clear: both" /> | ||
== HPLC analysis of analgesics == | == HPLC analysis of analgesics == | ||
| - | Another class of substrates were analgesics. | + | Another class of substrates we wanted to test were analgesics. The three analgesic substrates have different optimal extinction and emission values. Every analgesic had to be tested alone. With diclofenac the extinction and emission values were not found. Therefore the substrate was analyzed with the spectrofluorophotometer but this also showed no clear peak for diclofenac and made it therefore not measurable. Additional, difficulties occurred with ibuprofen. Instead of one single peak we found two and they didn't correlate with the used concentrations. |
| - | + | ||
| - | + | ||
| - | + | ||
<br style="clear: both" /> | <br style="clear: both" /> | ||
| - | + | = Outlook = | |
| - | Due to time reasons | + | The HPLC results showed that ECOL, BPUL, TTHL and BHAL are able to degrade estradiol in the presence and absence of ABTS. Ethinyl estradiol is not degraded by the bacterial laccases. Just TTHL showed little degradation activities on ethinyl estradiol in presence of ABTS. |
| + | Due to time reasons and the decay of PAHs in Britton Robinson buffer, the analyses of the PAHs and the analgesics with the HPLC and the LC-MS methods could not been carried out. It would be interesting to analyze the produced laccases with the PAHs and analgesics. | ||
| + | Degradation products were found after treatment of estradiol and ethinyl estradiol with TVEL0 with LC-MS. The next step would be to analyze the possible degradation of PAHs, analgesics and estrone and detect degradation products after treatment with the produced bacterial laccases and TVEL5 from ''Trametes versicolor''. | ||
| - | + | </div> | |
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<html> | <html> | ||
Latest revision as of 03:41, 27 October 2012

Contents |
Introduction
To investigate the degradation of different substrates with laccases several experiments were performed. For the measurements the four produced bacterial laccases (BHAL, ECOL, TTHL and BPUL) were used. The reactions were measured before and after an incubation with the laccases via high performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry. The HPLC was used particularly for analysis of degradation rates after defined time points. With these results it is possible to compare the different laccases in respect to their degradation feasibilities. To detect degradation products of estradiol and ethinyl estradiol after laccase treatment different analysis via LC-MS and LC-MS-MS were done. We identified two compounds for both, estradiol and ethinyl estradiol, which are probable degradation products after laccase treatment.
Degradation measurements with high performance liquid chromatography
Dilution series of different estrogens
At first dilution series of all different substrates were measured. It was possible to measure calibration curves for estradiol and ethinyl estradiol but not for estrone. This was probably caused by its bad solubility.
The retention time for estradiol is 4.4 minutes and for ethinyl estradiol 4.9 minutes. For all estrogens the same extinction and emission values could be used: Ex230, Em310.
Degradation of estrogens
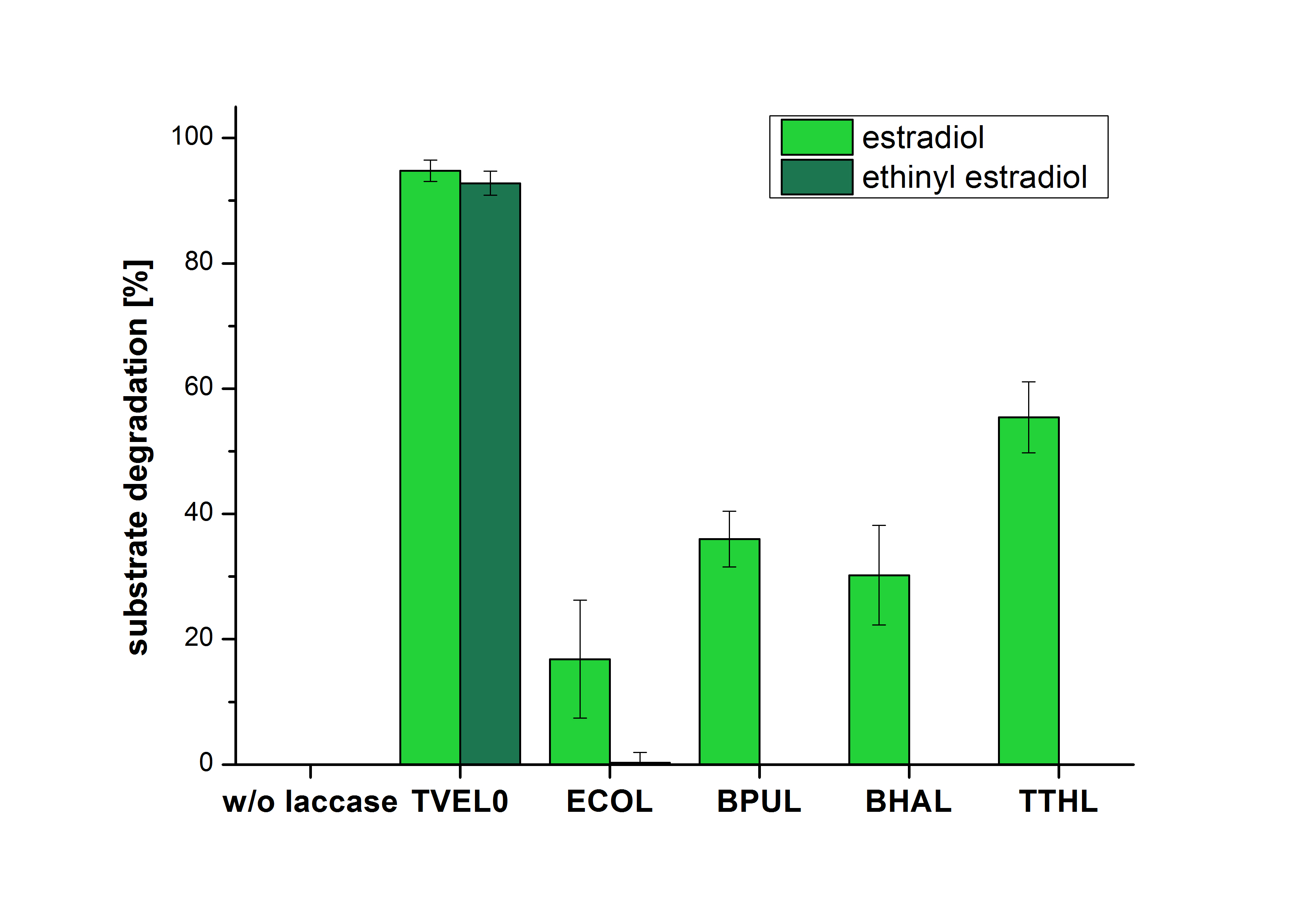
The measurements were made to test if the produced laccases were able to degrade different hormones. Therefore the produced laccases were inserted in the same concentrations (3 µg mL-1) to the different measurement approaches. To work with the correct pH value (which were measured by the Team Activity Test) Britton Robinson buffer at pH 5 was used for all measurements. The initial substrate concentration was 5 µg mL-1. The results of the reactions without ABTS are shown in Figure 2. On the Y-axis the percentages of degraded estradiol (blue) and ethinyl estradiol (red) are indicated. The X-axis displays the different tested laccases. The degradation was measured at t0 and after five hours of incubation at 30 °C. The negative control was the substrate in Britton Robinson buffer and showed no degradation of the substrates. The bought laccase TVEL0 which is used as positive control is able to degrade 94.7 % estradiol and 92.7 % ethinyl estradiol. The laccase BPUL (from Bacillus pumilus) degraded 35.9 % of used estradiol after five hours. ECOL was able to degrade 16.8 % estradiol. BHAL degraded 30.2 % estradiol. The best results were determined with TTHL (laccase from Thermus thermophilus). Here the percentage of degradation amounted 55.4 %.
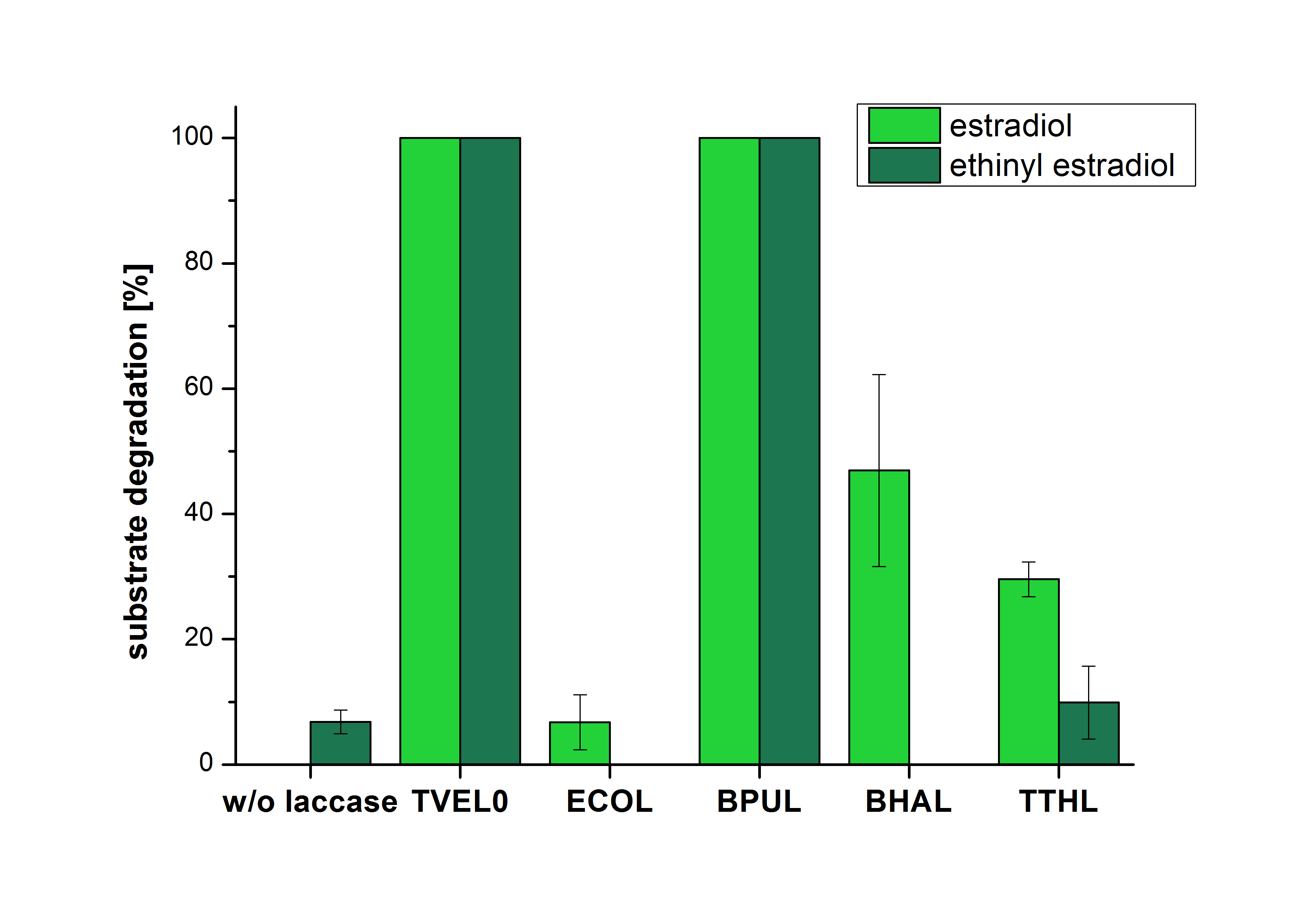
The results of the reactions of the laccases with addition of ABTS are shown in Figure 3. The experimental set ups were the same as the reaction approach without ABTS described above. The X-axis displays the different tested laccases. On the Y-axis the percentages of degraded estradiol (blue) and ethinyl estradiol (red) are shown. The degradation was measured at t0 and after five hours of incubation at 20 °C. The negative control showed no degradation of estradiol. 6.8 % of ethinyl estradiol was decayed. The positive control TVEL0 is able to degrade 100 % estradiol and ethinyl estradiol. The laccase BPUL (from Bacillus pumilus) degraded 46.9 % of used estradiol after ten minutes incubation. ECOL was able to degrade 6.7 % estradiol. BHAL degraded 46.9 % estradiol. With TTHL (laccase from Thermus thermophilus) a degradation 29.5 % were determined.
Spectrofluorophotometer Analysis
We analyzed the degradation of our substrates with a spectrofluorophotometer. As you can see in the figures below the ethinyl estradiol and estradiol are degraded over night. Figure 4 shows the ethinyl estradiol without laccase treatment, Figure 5 shows that no more ethinyl estradiol can be detected in the sample after the degradation and new peaks appear which might represent possible degradation products. In Figure 6 you can see the estradiol control without laccases. Like ethinyl estradiol theestradiol peak is reduced after the degradation and new peaks appear indicating that those are new degradation products.
Liquid chromatography–mass spectrometry
Dilution series
Our substrates are soluble in methanol. We set the standards to a concentration of 1 mg mL-1. The detection limit for the LC-MS was evaluated at a concentration of 10 µg L-1 for the substrates estrone and estradiol. The same limit of detection was used for ethinyl estradiol and anthracene. We only used those four substrates. For all LC-MS sample preparations we used the T. versicolor laccases. The dilution series was prepared in methanol and 50 % acetonitril-water (v/v).
Degradation results
The TVEL0 was able to degrade the synthetic estradiol (Fig. 10) and probably anthracene (Fig. 12). The ethinyl estradiol control showed that it is stable in the used media (Fig. 11). Anthracene disintegrates in the Britton Robinsoon Buffer. But it could be observed, that there is less anthracene measurable with the LC-MS. The results indicate, that the laccase is able to degrade anthracene (Fig. 13). Estrone (Fig. 14) and estradiol (Fig. 15) were degraded as well. Using estrone it could not be identify any degradation products. The reason for this could be that the products are not detectable with LC-MS or with the applied methods. Peaks in the degradation of estradiol have been shown but we were not able to identify them. It could be degradation products. In the following figures the results of the LC-MS measurements are presented.
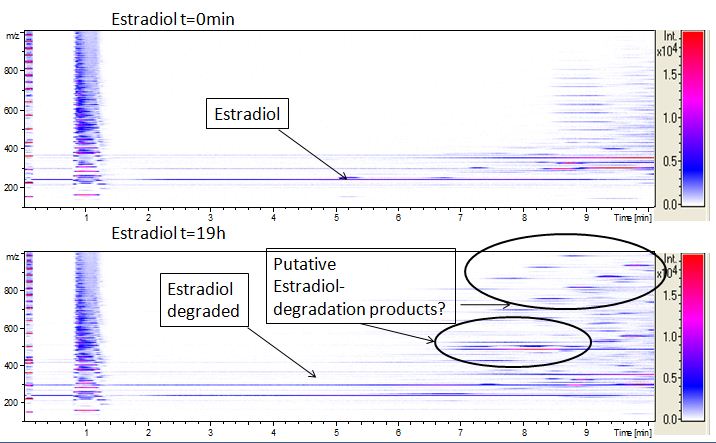
We also tried to measure the degradation using mass-spectrometry. Since quantification via mass-spectrometry is difficult regarding the ionization of the analytes, we quantified our substrates by UV-light. Nevertheless, mass spectrometry enables identification of possible degradation products. We analyzed estradiol degradation in detail (Fig. 15), resulting in the detection of possible chemical compounds generated during the (enzymatic) degradation.
Further analysis (after Regionals Amsterdam)
Since we have seen some possible degradation products, we used more estradiol, ethinyl estradiol (250µg L -1 compare to 50µg L -1 ) and more laccase (0,35U compare to 0,1U) for the reaction to the LC-MS measurement. We found out that after the laccase treatment two new peaks appeared in both the estradiol and ethinyl estradiol. A check against databases could not identify those components so we did an MS-MS on component A respectivly on component C. Since component B is fewer in concentration then component A, we could not find out anything about it (Fig. 16 and Fig. 17). To note the chemical formulares are M+H values. For the real chemical formula you have to deduct this H.
The tandem mass spectrometry results showed two peaks in every measurement for possible degradation products. Compared to the native estradiol the component A peak embodies a molecule with two hydrogen atoms less than the native, the same does component C for ethinly estradiol. These peaks were discussed in a short chat with Prof. Dr. Dietmar Kuck, an organic chemist with a lot experience in mass spectrometry. This conversation led to two plausible models for the degradation. The first was a reaction at the hydroxy group of the five-ring of the estradiol (Fig. 18), oxidizing it to a ketone (resulting in estrone as the degradation product). This idea seemed improbable, because ethinyl estradiol cannot be oxidized at that position, but its degradation product also had two hydrogen less than the native (it is impossible to oxidize the hydroxy group of the five-ring to a ketone without losing the ethinyl group or breaking the ring (Fig. 19)). The second model was that the laccase radicalizes the hydroxy group of the phenolic group of the estradiol and ethinyl estradiol, as described in literature, building a phenoxy radical in the first step. In the second step hydrogen is split off on the tertiary carbon in para position finally leading to a quinone like structure (Figure 19). Without knowing the mechanism behind this reaction, this seems the most probable model of the degradation.
The second peaks in the chromatograms (component B and D) embody molecules with two oxygen more and two hydrogen less than the native. We postulate that this might be a transition state where O2 is radically added to the phenolic ring in otho- and meta-position. To that point, there is no data to consolidate this adoption. Component B and D were also fragmented in the tandem mass spectrometry for further analysis, but the resulting peaks could not be differed from the background noise.
Further substrate analysis via HPLC
HPLC analysis of polycylic aromatic hydrocarbons
The results of our negative control measurements of polycyclic aromatic hydrocarbons showed that the PAHs decayed without laccase treatment after one hour in Britton Robinson (BR)-buffer. This is shown in Figure 20.
The next step was to check which substances in the reaction approach may cause the decay. Therefore naphthalene was dissolved in methanol (which is the solvent for the substrate and used for stopping the reaction), acetonitrile (which could be used as alternative solvent) and BR-buffer with and without ABTS. The results are shown in Figure 21. Using pure methanol and acetonitrile naphthalene is not decayed. In BR-buffer with and without ABTS the decay is nearly completed after one hour treatment. So BR-buffer seems a bad choice to test the degradation of naphtalene under laccase treatment.
HPLC analysis of analgesics
Another class of substrates we wanted to test were analgesics. The three analgesic substrates have different optimal extinction and emission values. Every analgesic had to be tested alone. With diclofenac the extinction and emission values were not found. Therefore the substrate was analyzed with the spectrofluorophotometer but this also showed no clear peak for diclofenac and made it therefore not measurable. Additional, difficulties occurred with ibuprofen. Instead of one single peak we found two and they didn't correlate with the used concentrations.
Outlook
The HPLC results showed that ECOL, BPUL, TTHL and BHAL are able to degrade estradiol in the presence and absence of ABTS. Ethinyl estradiol is not degraded by the bacterial laccases. Just TTHL showed little degradation activities on ethinyl estradiol in presence of ABTS. Due to time reasons and the decay of PAHs in Britton Robinson buffer, the analyses of the PAHs and the analgesics with the HPLC and the LC-MS methods could not been carried out. It would be interesting to analyze the produced laccases with the PAHs and analgesics. Degradation products were found after treatment of estradiol and ethinyl estradiol with TVEL0 with LC-MS. The next step would be to analyze the possible degradation of PAHs, analgesics and estrone and detect degradation products after treatment with the produced bacterial laccases and TVEL5 from Trametes versicolor.
| 55px | | | | | | | | | | |
 "
"