Team:University College London/Module 2/Results
From 2012.igem.org
Sednanalien (Talk | contribs) (→Shear Force Assay) |
Sednanalien (Talk | contribs) (→Congo Red Agar Assay) |
||
| (4 intermediate revisions not shown) | |||
| Line 12: | Line 12: | ||
{| class="wikitable" | {| class="wikitable" | ||
|+ Congo Red Agar Assay | |+ Congo Red Agar Assay | ||
| - | ! Control !! BBa_K729017 | + | ! Control (W3110) !! BBa_K729017 |
|- | |- | ||
| [[File:UniversityCollegeLondon_Curli_Congo_ Red_Control.jpg|475px]] || [[File:UniversityCollegeLondon_Curli_Congo_ Red_K729017.jpg|475px]] | | [[File:UniversityCollegeLondon_Curli_Congo_ Red_Control.jpg|475px]] || [[File:UniversityCollegeLondon_Curli_Congo_ Red_K729017.jpg|475px]] | ||
| Line 19: | Line 19: | ||
== Shear Force Assay == | == Shear Force Assay == | ||
| - | Using a shear device... | + | Using a rotary shear device, we have obtained data relating to the shear resistance of our curliated biofilms. Over a 24 hour period, biofilms were allowed to grow on the surface of plastic discs, which were then subjected to shear forces in the rotary shear device. By finding the critical shear radius, the point at which cells start to detach from the plastic discs, we have calculated the critical shear stresses that our biofilms are capable of resisting. |
| - | Based on the results we have obtained, we have determined that the biofilms produced by cells transformed with BBa_K729018 have a two-fold shear resistance | + | Based on the results we have obtained, we have determined that the biofilms produced by cells transformed with BBa_K729018 have a two-fold shear resistance when compared to the biofilms of the control cell line W3110. This gives corroborating evidence that our curli BioBrick is functioning as intended, as curli generated biofilms are more resistant to shear forces, and detach less readily. |
[[File:UniversityCollegeLondon_Curli_Shear_Stress.png|450px|centre]] | [[File:UniversityCollegeLondon_Curli_Shear_Stress.png|450px|centre]] | ||
Latest revision as of 02:37, 27 September 2012
Module 2: Aggregation
Description | Design | Construction | Characterisation | Shear Device | Modelling | Results | Conclusions
Congo Red Agar Assay
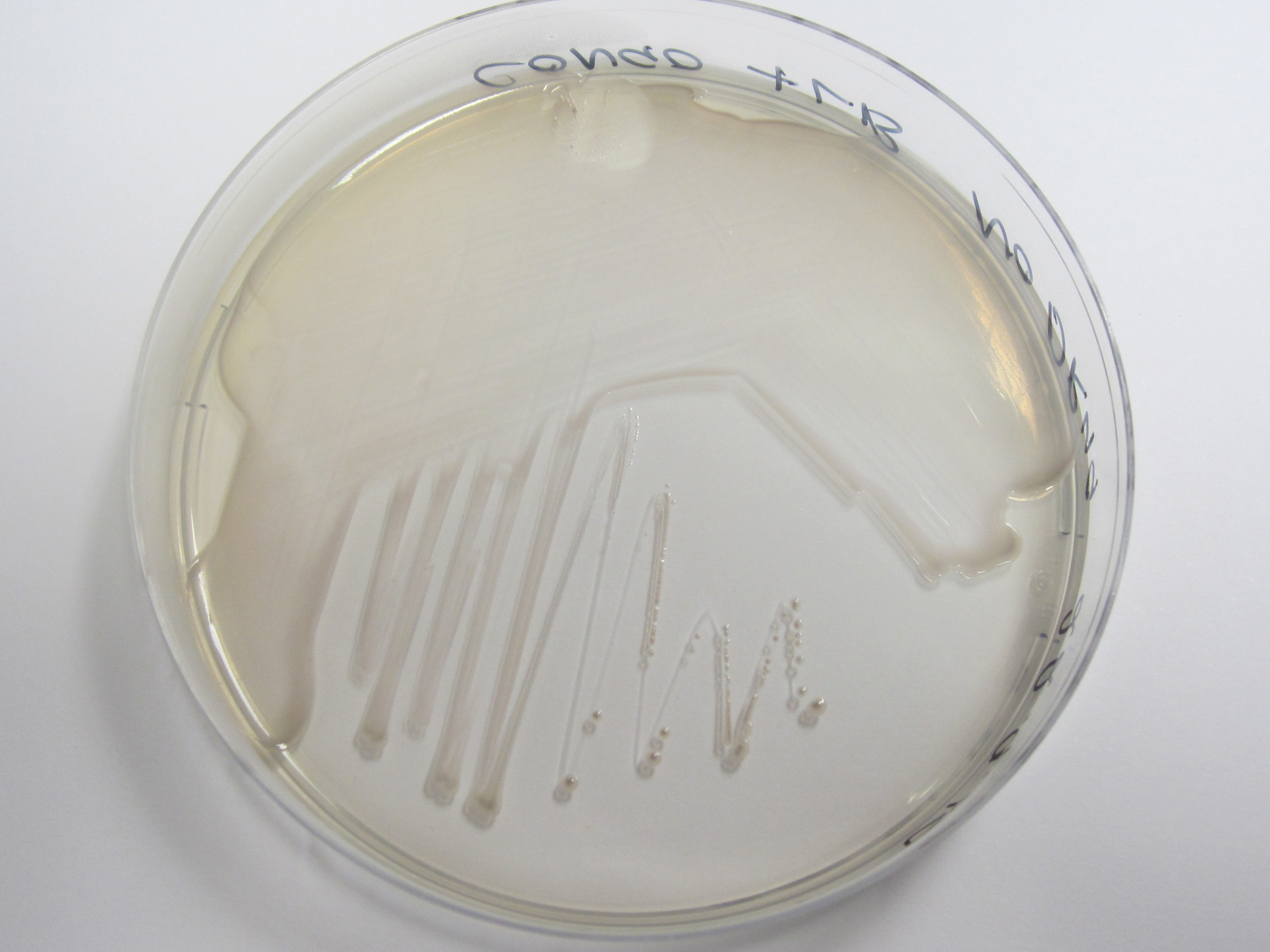
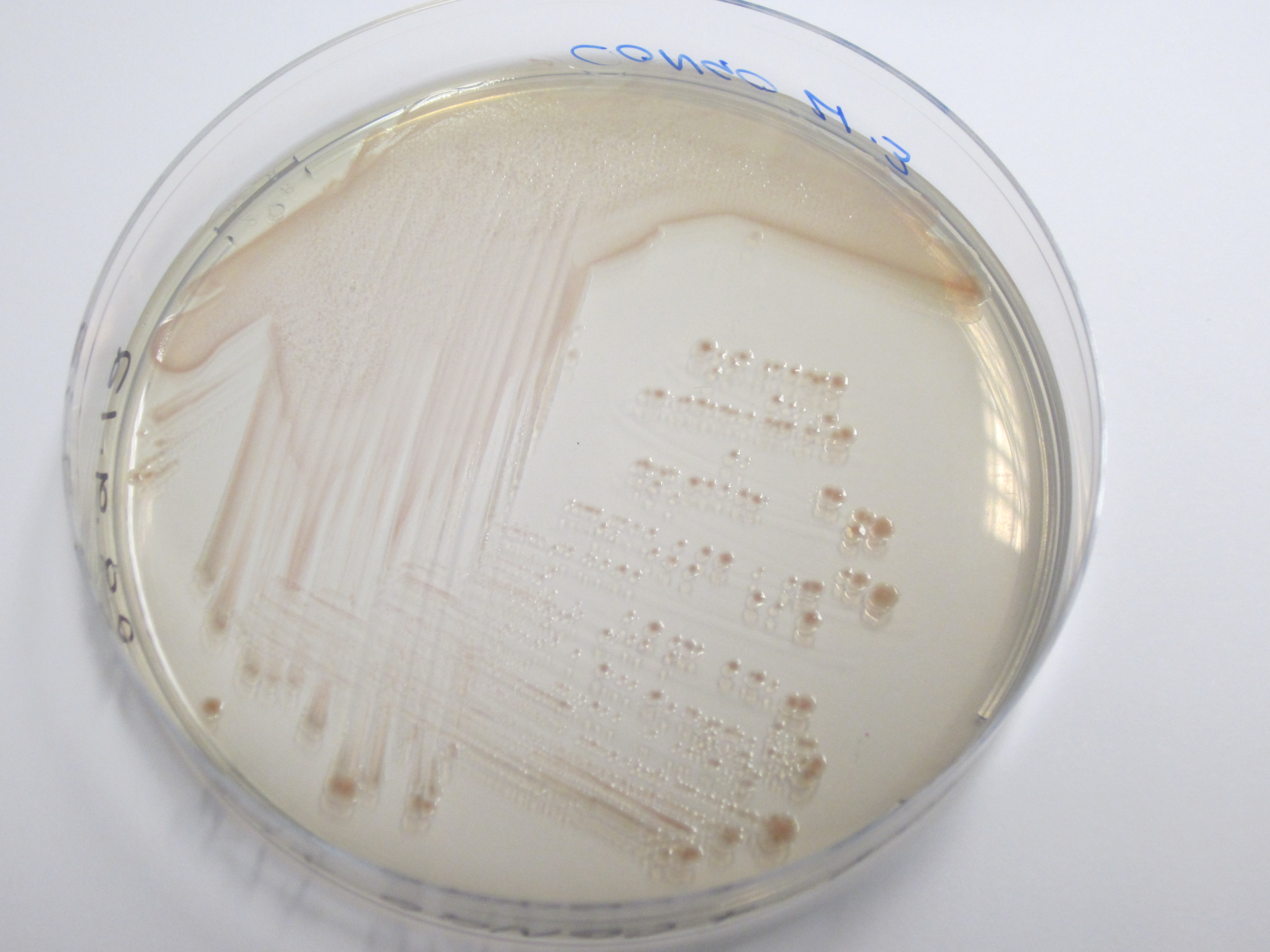
We have used a congo red agar assay to determine if our curli BioBrick transformed cells have successfully expressed the amyloid fibrins. Congo red is a diazo dye that binds to the curli fibrins, thereby causing cells positive for curlis to appear red when grown on this agar.
As can be seen from plates below, our cells transformed with BBa_K729017 display a red tinge to their colonies, while the untransformed W3110 cells do not. We hence conclude that we have successfully transformed our cells to produce curlis.
| Control (W3110) | BBa_K729017 |
|---|---|
 | 
|
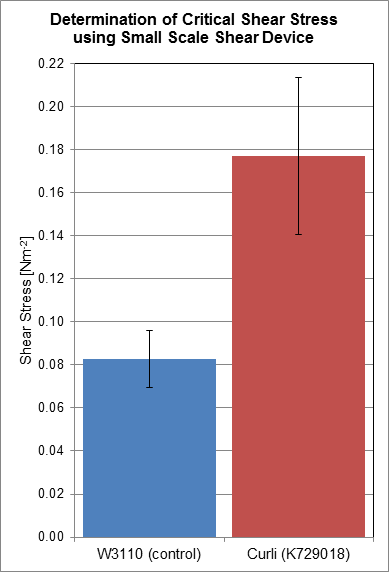
Shear Force Assay
Using a rotary shear device, we have obtained data relating to the shear resistance of our curliated biofilms. Over a 24 hour period, biofilms were allowed to grow on the surface of plastic discs, which were then subjected to shear forces in the rotary shear device. By finding the critical shear radius, the point at which cells start to detach from the plastic discs, we have calculated the critical shear stresses that our biofilms are capable of resisting.
Based on the results we have obtained, we have determined that the biofilms produced by cells transformed with BBa_K729018 have a two-fold shear resistance when compared to the biofilms of the control cell line W3110. This gives corroborating evidence that our curli BioBrick is functioning as intended, as curli generated biofilms are more resistant to shear forces, and detach less readily.
 "
"