Team:HokkaidoU Japan/Notebook/aggregation Week 5
From 2012.igem.org
| Line 201: | Line 201: | ||
</p> | </p> | ||
| + | |||
| + | ==Electrophoresis== | ||
| + | Electrophoresis of digestion result of yesterday(pT7-RBS on pSB1K3 cut with SpeI) | ||
| + | |||
| + | [[image:HokkaidoU2012 120802 pT7-RBS on pSB1K3 SpeI.jpg|thumb|digestion result]] | ||
| + | There were low concentration band in above of thin band. This thin band was same as digestion minus band. | ||
| + | |||
</div></div> | </div></div> | ||
Revision as of 10:47, 2 August 2012
Contents |
July 30th
digestion
I tried to digest the mini-prep products again, and after Ethanol precipitation, we did electrophoresis.
transformation
I think that the E. coli which we use transformation is BL21(DE3)pLysS.
So, the E. coli could multiplication increase on LBC plate.
I'm going to do transformation , use DH5α.
Transformation of plasmid DNA ligated in 25th (pT7-RBS-Ag43-dT on pSB1K3) in E. coli(DH5α).
- Added 2ul of plasmid DNA to 50ul of thawed competent cells on ice.
- Incubated on ice for 30min.
- Added 200ul of LB then incubated the cells for 2 hours at 37C.
- Prepared and Labeled two petri dishes with LBK.
- Plate 200ul of the transformation onto first dish and spread.
- Added 450ul of LB to 50ul of the transformation and plated 200ul of it onto second dish and spread.
- Incubated the plates at 37C for 14 hours.
July 31th
liquid culture
Liquid culture for pT7-RBS-Ag43-dT on pSB1K3.
- Added 2ml of LBK into culture tubes.
- Resuspended colonies.
- Incubated the tubes at 37C for
August 1st
mini-prep
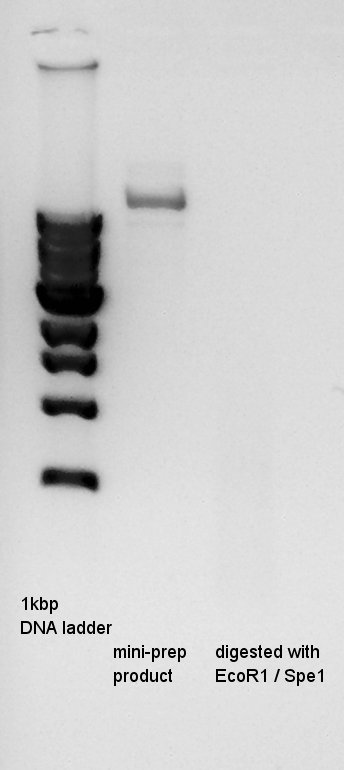
mini-prep of pT7-RBS-Ag43-dT which had been cultivated from yesterday. We used FastGene Plasmid Mini Kit(Nippon Genetics) and got 50ul of DNA solutions. [[image:|thumb|mini-prep result]]
Digestion
pT7-RBS(20ng/ul) =⑨ SpeI 10xH
| DNA solution | 4.5ul |
| SpeI | 1ul |
| 10xH buffer | 1ul |
| DW | 3.5ul |
| Total | 10ul |
SpeI 10xM
| DNA solution | 4.5ul |
| SpeI | 1ul |
| 10xM buffer | 1ul |
| DW | 3.5ul |
| Total | 10ul |
pT7-RBS(30ng/ul) =⑩
SpeI 10xH
| DNA solution | 3ul |
| SpeI | 1ul |
| 10xH buffer | 1ul |
| DW | 5ul |
| Total | 10ul |
SpeI 10xM
| DNA solution | 3ul |
| SpeI | 1ul |
| 10xM buffer | 1ul |
| DW | 5ul |
| Total | 10ul |
[[image:|thumb|digestion result]]
We couldn't cut them exactry so we cut once more time.
pT7-RBS(20ng/ul) =⑨ SpeI 10xH
| DNA solution | 4.5ul |
| SpeI | 1ul |
| 10xM buffer | 2ul |
| DW | 12.5ul |
| Total | 20ul |
Liquid culture
Liquid culture for pBAD-RBS on pSB1K3.
- Added 2ml of LBK into culture tube.
- Scraped the surface of glycerol stock of construct.
- Incubated the tube at 33C for OOhrs.
August 2nd
Preparing chemical competent cell
Preparing chemical competent cell of BL21, JM109 and DH5α. Chemical competent cell made in each E.coli strains.
Our competent cell Protocol
- Single colony isolation on LB plate
- incubate the plate for 15-19 hours at 37℃
- lift colony of E.coli into 2 ml LB
- culture cells at 37℃ for 12-16 hours at 180-200 rpm
- transfer 30 ul, 100 ul, 300 ul of the culture into 100 ml of SOB medium, respectively
- culture cells at 20℃ (for 24 hours over) at 180-200 rpm (to ΔOD550nm=0.5~0.6)
- leave the 300 ml flask for 10 min on ice
- transfer the culture into two 50 ml Falcon tube
- centrifuge 3000 rpm at 4℃ for 20 min (TOMY LX-120 rotor), and discard sup
- suspend the pellet in ice-cold 15 ml of TB (Transformation Buffer)(7.5 ml/tube)
- collect them in one tube
- centrifuge 3000 rpm at 4℃ for 20 min (TOMY LX-120 rotor), and discard sup
- suspend the pellet in ice-cold 3.2 ml of TB (Transformation Buffer)
- Instil 0.24 ml of DMSO in precipitant
- leave the 50 ml Falcon tube for 10 min on ice
- divide 50ul of solutions in each 1.5 or 0.5 ml tubes
- Freeze the suspension in liquid nitrogen
- store at –80℃
Electrophoresis
Electrophoresis of digestion result of yesterday(pT7-RBS on pSB1K3 cut with SpeI)
There were low concentration band in above of thin band. This thin band was same as digestion minus band.
 "
"