Team:HokkaidoU Japan/Notebook/Week 3
From 2012.igem.org
(Gel Extraction, Ethanol precipitation) |
|||
| Line 5: | Line 5: | ||
==July 16th== | ==July 16th== | ||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| + | Ag43, dT | ||
===Digestion=== | ===Digestion=== | ||
Results of digestion in 15th. | Results of digestion in 15th. | ||
| Line 25: | Line 26: | ||
#Centrifuged in 15000rpm, 10min at 4C. | #Centrifuged in 15000rpm, 10min at 4C. | ||
#Remove supernatant and air drying in room temperature then added 5ul of DW. | #Remove supernatant and air drying in room temperature then added 5ul of DW. | ||
| + | |||
| + | ---- | ||
| + | |||
| + | dT(B0015) would be amplified incorrectly. So we tried another DNA amplification method: PCR. | ||
| + | |||
| + | ===PCR=== | ||
| + | PCR for dT(B0015) | ||
| + | |||
| + | {|class="hokkaidou-table-pcr-reagent" | ||
| + | |- | ||
| + | |DNA solution | ||
| + | |1ul | ||
| + | |- | ||
| + | |KOD-Plus-NEO(Taq polymerase) | ||
| + | |1ul | ||
| + | |- | ||
| + | |dNTP | ||
| + | |5ul | ||
| + | |- | ||
| + | |MgSO4 | ||
| + | |3ul | ||
| + | |- | ||
| + | |KOD-Plus-NEO Buffer | ||
| + | |5ul | ||
| + | |- | ||
| + | |Forward Primer(100bp_up forward primer) | ||
| + | |1ul | ||
| + | |- | ||
| + | |Reverse Primer(200bp_down Reverse primer) | ||
| + | |1ul | ||
| + | |- | ||
| + | |DW | ||
| + | |33ul | ||
| + | |- | ||
| + | |Total | ||
| + | |50ul | ||
| + | |} | ||
| + | |||
| + | |||
| + | {|class="hokkaidou-table-pcr-time" | ||
| + | |- | ||
| + | |Number | ||
| + | |Degree | ||
| + | |Second | ||
| + | |- | ||
| + | |1 | ||
| + | |94 | ||
| + | |120 | ||
| + | |- | ||
| + | |2 | ||
| + | |98 | ||
| + | |10 | ||
| + | |- | ||
| + | |3 | ||
| + | |58 | ||
| + | |30 | ||
| + | |- | ||
| + | |4 | ||
| + | |68 | ||
| + | |30 | ||
| + | |- | ||
| + | |5 | ||
| + | |4 | ||
| + | |HOLD | ||
| + | |} | ||
| + | Cycle:2~4 x 45 | ||
| + | |||
| + | |||
| + | |||
| + | [[image:HokkaidoU2012 120716-dt-ethandpcr.jpg|thumb|PCR result image]] | ||
| + | |||
| + | We migrated B0015 mini-prep psoduct, digestion product, and PCR product. | ||
| + | PCRed dT would have 429bp(100bp(added by forward primer) + 129bp(dT) + 200bp(added by reverse primer)). Most bright and thick band in this image has about 300~400 bp. We thought dT was amplified successfully. | ||
| + | |||
| + | |||
| + | ---- | ||
| + | |||
| + | Ag43 | ||
| + | |||
| + | Digestion result of Ag43 was incorrect. We digested Ag43 once more time. | ||
| + | |||
| + | ===Digestion=== | ||
| + | Digestion for Ag43 with SpeI and PstI. | ||
| + | |||
| + | {|class="hokkaidou-table-digestion" | ||
| + | |- | ||
| + | |Ag43 DNA solution | ||
| + | |9ul | ||
| + | |- | ||
| + | |SpeI | ||
| + | |1ul | ||
| + | |- | ||
| + | |PstI | ||
| + | |1ul | ||
| + | |- | ||
| + | |10xH buffer | ||
| + | |2ul | ||
| + | |- | ||
| + | |DW | ||
| + | |7ul | ||
| + | |- | ||
| + | |Total | ||
| + | |20ul | ||
| + | |} | ||
| + | |||
| + | |||
| + | [[image:|thumb|Digestion result image]] | ||
Revision as of 11:40, 16 July 2012
Contents |
July 16th
Ag43, dT
Digestion
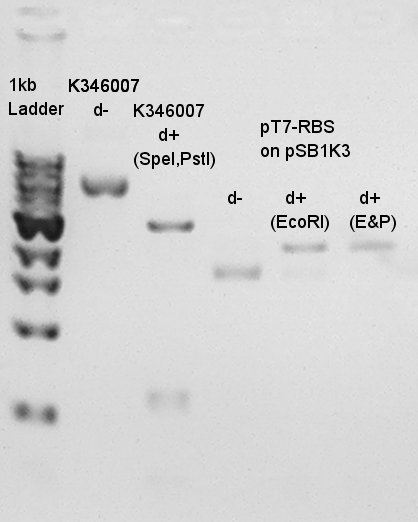
Results of digestion in 15th.
Product:Ag43(K346007)=3120bp and 2070bp, pT7-RBS on pSB1K3=2247bp We confirmed there are some kind of restriction enzyme site in K346007 (digested with SpeI, PstI) and pT7-RBS on pSB1K3 was successfully digested with EcoRI and PstI. Balance between d+(EcoRI) and d+(E&P:cut with EcoRI and PstI) is about 80bp.
Gel Extraction
Gel Extraction for digestion products. We used FastGene Gel&PCR extraction kit(NipponGenetics). Got 50ul of DNA solution.
Ethanol Precipitation
Ethanol Precipitation for digestion and gel extraction products.
- Added 5ul of NaoAc, 1.5ul of glycogen and 125ul of 100% ethanol.
- Centrifuged in 15000rpm, 10min at 4C.
- Remove supernatant and added 220ul of 70% ethanol.
- Centrifuged in 15000rpm, 10min at 4C.
- Remove supernatant and air drying in room temperature then added 5ul of DW.
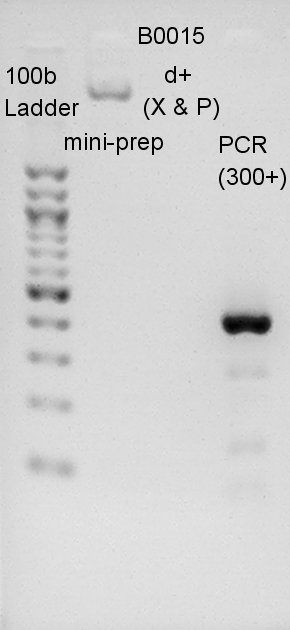
dT(B0015) would be amplified incorrectly. So we tried another DNA amplification method: PCR.
PCR
PCR for dT(B0015)
| DNA solution | 1ul |
| KOD-Plus-NEO(Taq polymerase) | 1ul |
| dNTP | 5ul |
| MgSO4 | 3ul |
| KOD-Plus-NEO Buffer | 5ul |
| Forward Primer(100bp_up forward primer) | 1ul |
| Reverse Primer(200bp_down Reverse primer) | 1ul |
| DW | 33ul |
| Total | 50ul |
| Number | Degree | Second |
| 1 | 94 | 120 |
| 2 | 98 | 10 |
| 3 | 58 | 30 |
| 4 | 68 | 30 |
| 5 | 4 | HOLD |
Cycle:2~4 x 45
We migrated B0015 mini-prep psoduct, digestion product, and PCR product. PCRed dT would have 429bp(100bp(added by forward primer) + 129bp(dT) + 200bp(added by reverse primer)). Most bright and thick band in this image has about 300~400 bp. We thought dT was amplified successfully.
Ag43
Digestion result of Ag43 was incorrect. We digested Ag43 once more time.
Digestion
Digestion for Ag43 with SpeI and PstI.
| Ag43 DNA solution | 9ul |
| SpeI | 1ul |
| PstI | 1ul |
| 10xH buffer | 2ul |
| DW | 7ul |
| Total | 20ul |
[[image:|thumb|Digestion result image]]
 "
"