Team:NTU-Taida/Project/Effector
From 2012.igem.org
(→Glucagon-like Peptide-1) |
|||
| (26 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{:Team:NTU-Taida/Templates/Header}}{{:Team:NTU-Taida/Templates/Navbar}}{{:Team:NTU-Taida/Templates/Sidebar|Title=Effector}}{{:Team:NTU-Taida/Templates/ContentStart}} | {{:Team:NTU-Taida/Templates/Header}}{{:Team:NTU-Taida/Templates/Navbar}}{{:Team:NTU-Taida/Templates/Sidebar|Title=Effector}}{{:Team:NTU-Taida/Templates/ContentStart}} | ||
| - | {{:Team:NTU-Taida/Templates/BSHero|Title=Effector|Content=}} | + | {{:Team:NTU-Taida/Templates/BSHero|Title=Effector|Content=<p>We use neuropeptide GLP-1 as the effector. Targeting to the brain, it can modulate the eating behavior of hosts and has neuroprotective function as well. Cell penetrating peptide and signal sequence are designed for facilitation of penetration and secretion, respectively.</p>}} |
==Glucagon-like Peptide-1== | ==Glucagon-like Peptide-1== | ||
| - | '''Glucagon-like Peptide-1 (GLP-1)''' is one of the members of '''incretin''', a group of gastrointestinal hormones secreted by endocrine cells in small intestine that mainly work to increase insulin levels. Under normal physiological condition, this 30-amino acid peptide is secreted by L cells, a kind of neuroendocrine cell located in small intestine after a meal, into the circulation, promoting insulin release and inhibit energy intake. Its natural form can be degraded by DPP-4, having a half life of 1-2 minutes. GLP-1 mainly acts on the '''GLP-1 receptor''', a G-protein coupled receptors, coupled with adenylyl cyclase downstream and signaling on PI3K/Akt and ERK1/2 pathways via second messenger cAMP. It promotes the secretion of insulin in β cells of islets in pancreas, antagonizes and glucose-rising effect of glucagon, and largely increase the insulin sensitivity of targeted cells. GLP-1 analogues such as Liraglutide and Exentide and DPP-4 inhibitors have been used clinically as treatment for patients with '''type 2 diabetes mellitus (T2DM)''', but recently, more and more focus has been spotted on its versatile functions, such as cardiovascular regulation, appetite inhibition, and neuroprotective effects. Here we aim to utilize the functions in appetite inhibition and neuroprotection of GLP-1, in order to tailor our PEPDEX as a living machine that communicate with our central nervous systems and modulate our eating behavior, as well as improve our cognitive function to prevent neurodegenerative diseases. | + | <p style="text-indent: 2em;">'''Glucagon-like Peptide-1 (GLP-1)''' is one of the members of '''incretin''', a group of gastrointestinal hormones secreted by endocrine cells in small intestine that mainly work to increase insulin levels. Under normal physiological condition, this 30-amino acid peptide is secreted by L cells, a kind of neuroendocrine cell located in small intestine after a meal, into the circulation, promoting insulin release and inhibit energy intake. Its natural form can be degraded by DPP-4, having a half life of 1-2 minutes. GLP-1 mainly acts on the '''GLP-1 receptor''', a G-protein coupled receptors, coupled with adenylyl cyclase downstream and signaling on PI3K/Akt and ERK1/2 pathways via second messenger cAMP. It promotes the secretion of insulin in β cells of islets in pancreas, antagonizes and glucose-rising effect of glucagon, and largely increase the insulin sensitivity of targeted cells. GLP-1 analogues such as Liraglutide and Exentide and DPP-4 inhibitors have been used clinically as treatment for patients with '''type 2 diabetes mellitus (T2DM)''', but recently, more and more focus has been spotted on its versatile functions, such as cardiovascular regulation, appetite inhibition, and neuroprotective effects. Here we aim to utilize the functions in appetite inhibition and neuroprotection of GLP-1, in order to tailor our PEPDEX as a living machine that communicate with our central nervous systems and modulate our eating behavior, as well as improve our cognitive function to prevent neurodegenerative diseases.</p> |
| - | [[FIle:NTU-Taida-Project-GLP-1. | + | [[FIle:NTU-Taida-Project-GLP-1.jpg|500px|thumb|center|Versatile function of GLP-1]] |
| - | ==Fat Extinguisher—Reduction in Eating Behavior== | + | ===Fat Extinguisher—Reduction in Eating Behavior=== |
Obesity and metabolic syndrome have been regarded as chronic disease recent decades. It is reported that people with obesity or metabolic syndromes are in a higher risk of type II diabetes, cardiovascular disease and hypertension. Nowadays, there are 1 billion people encountering over-weight problem, and 30 million people are diagnosed of obesity. Modern dietary pattern has been considered the major cause contributing to the phenomena. | Obesity and metabolic syndrome have been regarded as chronic disease recent decades. It is reported that people with obesity or metabolic syndromes are in a higher risk of type II diabetes, cardiovascular disease and hypertension. Nowadays, there are 1 billion people encountering over-weight problem, and 30 million people are diagnosed of obesity. Modern dietary pattern has been considered the major cause contributing to the phenomena. | ||
| - | GLP-1 is known to act on the satiety center inside hypothalamic regions of brains, inhibiting appetite and modulating feeding behavior. Therapeutic use of anti-obesity has also been proved by different drug authorities. In PepdEx, we put GLP-1 production under the control of a circuit with a fatty acid sensor, mimicking the physiological response that GLP-1 is released after food intake. Induced by a fat meal, bacterial secreted GLP-1 are expected to penetrate the intestinal epithelium with the assistance of cell penetrating peptide Penetratin, circulating to the brain and perform its behavior-regulating function. | + | GLP-1 is known to act on the '''satiety center''' inside '''hypothalamic regions''' of brains, inhibiting '''appetite''' and modulating '''feeding behavior'''. Therapeutic use of anti-obesity has also been proved by different drug authorities. In PepdEx, we put GLP-1 production under the control of a circuit with a fatty acid sensor, mimicking the physiological response that GLP-1 is released after food intake. Induced by a fat meal, bacterial secreted GLP-1 are expected to penetrate the intestinal epithelium with the assistance of cell penetrating peptide '''Penetratin''', circulating to the brain and perform its behavior-regulating function. |
| - | ==Brain Guardian—Memory Improvement and Neuroprotection == | + | ===Brain Guardian—Memory Improvement and Neuroprotection === |
| - | Besides regulation of appetite, GLP-1 also has wide effects on the central nervous system, including promotion of learning and memory and neuroprotective roles. GLP-1 receptors are distributed among the brain in various regions such as hypothalamus, thalamus, brainstem, and hippocampus. Studies using in vitro culture and animal models indicate that GLP-1 promote the learning and memory function in CA1 region of hippocampus, via increase of long-term potentiation (LTP) and enhancement of synaptic plasticity. It also reduces the destructive effects of Aβ amyloid and preserve and dopaminergic neurons in Alzheimer’s disease and Parkinson’s disease, respectively. Potentiation of neuronal differentiation, promotion of cell survival, up-regulation of neurotransmitter synthesis and modulation of glial cells are other functions of GLP-1 in CNS that have been reported, while more diseases such as Huntington’s disease, stroke, and peripheral sensory neuropathy potentially become its therapeutic targets. Conjugation with a thermal regulatory promoter gives a constitutive delivery of GLP-1 inside the human body, which constantly stimulates and protects the CNS, in improvement of learning and memory and prevention of neurodegenerative diseases. | + | Besides regulation of appetite, GLP-1 also has wide effects on the central nervous system, including promotion of learning and memory and neuroprotective roles. GLP-1 receptors are distributed among the brain in various regions such as '''hypothalamus''', '''thalamus''', '''brainstem''', and '''hippocampus'''. Studies using in vitro culture and animal models indicate that GLP-1 promote the learning and memory function in CA1 region of hippocampus, via increase of '''long-term potentiation (LTP)''' and enhancement of '''synaptic plasticity'''. It also reduces the destructive effects of Aβ amyloid and preserve and dopaminergic neurons in '''Alzheimer’s disease''' and '''Parkinson’s disease''', respectively. Potentiation of neuronal differentiation, promotion of cell survival, up-regulation of neurotransmitter synthesis and modulation of glial cells are other functions of GLP-1 in CNS that have been reported, while more diseases such as '''Huntington’s disease''', '''stroke''', and '''peripheral sensory neuropathy''' potentially become its therapeutic targets. Conjugation with a thermal regulatory promoter gives a constitutive delivery of GLP-1 inside the human body, which constantly stimulates and protects the CNS, in improvement of learning and memory and prevention of neurodegenerative diseases. |
<ol> | <ol> | ||
<li>De Silva A, et al. (2011) The Gut Hormones PYY3-36 and GLP-17-36 amide Reduce Food Intake and Modulate Brain Activity in Appetite Centers in Humans. Cell Metab '''14(5)''':700-6.</li> | <li>De Silva A, et al. (2011) The Gut Hormones PYY3-36 and GLP-17-36 amide Reduce Food Intake and Modulate Brain Activity in Appetite Centers in Humans. Cell Metab '''14(5)''':700-6.</li> | ||
| Line 40: | Line 40: | ||
==Cell Penetrating Peptide == | ==Cell Penetrating Peptide == | ||
| - | One obstacle needs to be overcome is the poor penetrating ability of GLP-1 through the intestinal epithelia, since GLP-1 is neither small molecule nor hydrophobic. However, several studies have shown that some short peptides known as cell penetrating peptides (CPPs) can help to deliver biodrugs through intestinal mucosa and increase their bioavailability. CPPs are usually arginine-rich, tend to interact with extracellular matrix and can enhance macropinocytosis of epithelial cells. One recent study even demonstrated that co-administration of GLP-1 and Penetratin, one of the CPPs, greatly increased the intestinal absorption rate to 5%. By applying cell penetrating peptide to our device, we aim to promote the delivery of GLP-1 from intestinal lumen to blood stream, therefore enhance their appetite-regulating effect. | + | One obstacle needs to be overcome is the poor penetrating ability of GLP-1 through the intestinal epithelia, since GLP-1 is neither small molecule nor hydrophobic. However, several studies have shown that some short peptides known as '''cell penetrating peptides (CPPs)''' can help to deliver biodrugs through intestinal mucosa and increase their bioavailability. CPPs are usually arginine-rich, tend to interact with extracellular matrix and can enhance macropinocytosis of epithelial cells. One recent study even demonstrated that co-administration of GLP-1 and '''Penetratin''', one of the CPPs, greatly increased the intestinal absorption rate to 5%. By applying cell penetrating peptide to our device, we aim to promote the delivery of GLP-1 from intestinal lumen to blood stream, therefore enhance their appetite-regulating effect. |
| + | [[FIle:NTU-Taida-Project-CPP.png|500px|thumb|center|Transepithelial effect of CPP]] | ||
| + | |||
| + | <html> | ||
| + | <table class='table table-bordered table-hover'> | ||
| + | <tr> | ||
| + | <td><b>Peptides</b></td> | ||
| + | <td><b>Sequence</b></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><i>Arginine rich peptides</i></td> | ||
| + | <td></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Arginine octamer (R8)</td> | ||
| + | <td>RRRRRRRR</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Arginine dodecamer (R12)</td> | ||
| + | <td>RRRRRRRRRRRR</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>HIV-1 Tat (48–60)</td> | ||
| + | <td>GRKKRRQRRRPPQ</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>HIV-1 Rev (34–50)</td> | ||
| + | <td>TRQARRNRRRRWRERQR</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><i>Amphipathic peptides</i></td> | ||
| + | <td></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><b>Penetratin</b></td> | ||
| + | <td><b>RQIKIWFQNRRMKWKK</b></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pVEC</td> | ||
| + | <td>LLIILRRRIRKQAHAHSK</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Erns</td> | ||
| + | <td>RQGAARVTSWLGLQLRIGK</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>RRL helix</td> | ||
| + | <td>RRLRRLLRRLRRLLRRLR</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>PRL4</td> | ||
| + | <td>PRLPRLPRLPRL</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><i>Random composition peptides</i></td> | ||
| + | <td></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Random peptide</td> | ||
| + | <td>GLSASPNLQFRTV</td> | ||
| + | </tr> | ||
| + | |||
| + | |||
| + | |||
| + | </table> | ||
| + | </html> | ||
| + | |||
| + | |||
<ol> | <ol> | ||
<li>Khafagy el-S, et al. (2012) Oral biodrug delivery using cell-penetrating peptide. Adv Drug Deliv Rev '''64(6)''':531-9.</li> | <li>Khafagy el-S, et al. (2012) Oral biodrug delivery using cell-penetrating peptide. Adv Drug Deliv Rev '''64(6)''':531-9.</li> | ||
| Line 50: | Line 117: | ||
In order to achieve the extracellular secretion of our recombinant peptide GLP-1 and CPP, we utilize the endogenous type 2 secretion system (T2SS) in bacteria with specific signal peptides added to the N-terminus of GLP-1 and CPP. | In order to achieve the extracellular secretion of our recombinant peptide GLP-1 and CPP, we utilize the endogenous type 2 secretion system (T2SS) in bacteria with specific signal peptides added to the N-terminus of GLP-1 and CPP. | ||
| - | ==Type 2 Secretion System== | + | ===Type 2 Secretion System=== |
Secretion systems of gram-negative bacteria are categorized into 6 types, from type 1 secretion system (T1SS) to type 6 secretion system (T6SS). Among these secretion systems, type 1 and type 2 secretion systems are in charge of transport bacterial protein products into the extracellular space. [[ #Ref | [1] ]] Here we choose type 2 secretion system (T2SS) as our secretion apparatus which is widely found in gram-negative bacteria, including E. coli. Unlike the single-step secretion of T1SS such as hemolysin transport system (Hly), T2SS has two-step secretion processes. Unfolded or folded proteins with specific signal sequences on their N-terminus are first transported through the inner membrane into the periplasmic space via Sec translocon or twin-arginine translocation (Tat) pathway, respectively. [[ #Ref | [1, 4, 5] ]] The cleavage of signal peptides is performed by signal peptidase inside the periplasm. Proteins of proper structural features are then transported through the outer membrane into the extracellular space via general secretory pathway (GSP) which consists of 4 distinguished subassemblies: inner membrane platform, outer membrane complex, pseudopilus, and secretion ATPase (Fig. 1) [[ #Ref | [2, 3] ]], while small proteins (in our case, short peptides) can also leak from the periplasmic into the environment, regarding the integrity of the outer membrane. Co-expressions of some genes, such as kil, out, tolAIII, and bacteriocin release protein (BRP), were reported to facilitate the extracellular secretory processes. [[ #Ref | [1] ]] | Secretion systems of gram-negative bacteria are categorized into 6 types, from type 1 secretion system (T1SS) to type 6 secretion system (T6SS). Among these secretion systems, type 1 and type 2 secretion systems are in charge of transport bacterial protein products into the extracellular space. [[ #Ref | [1] ]] Here we choose type 2 secretion system (T2SS) as our secretion apparatus which is widely found in gram-negative bacteria, including E. coli. Unlike the single-step secretion of T1SS such as hemolysin transport system (Hly), T2SS has two-step secretion processes. Unfolded or folded proteins with specific signal sequences on their N-terminus are first transported through the inner membrane into the periplasmic space via Sec translocon or twin-arginine translocation (Tat) pathway, respectively. [[ #Ref | [1, 4, 5] ]] The cleavage of signal peptides is performed by signal peptidase inside the periplasm. Proteins of proper structural features are then transported through the outer membrane into the extracellular space via general secretory pathway (GSP) which consists of 4 distinguished subassemblies: inner membrane platform, outer membrane complex, pseudopilus, and secretion ATPase (Fig. 1) [[ #Ref | [2, 3] ]], while small proteins (in our case, short peptides) can also leak from the periplasmic into the environment, regarding the integrity of the outer membrane. Co-expressions of some genes, such as kil, out, tolAIII, and bacteriocin release protein (BRP), were reported to facilitate the extracellular secretory processes. [[ #Ref | [1] ]] | ||
| + | [[FIle:NTU-Taida-Project-T2SS.png|600px|thumb|center|Summary of Type 2 Secretion System]] | ||
| - | ==Signal Peptides== | + | ===Signal Peptides=== |
The selection of signal sequence for delivery of specific recombinant proteins is mostly based on empirical data. Some representative signal sequences, such as those of PhoA (alkaline phosphatase), PelB (pectate lysase B), OmpA (outer-membrane protein A), OmpF (outer-membrane protein F), StII (heat-stable enterotoxin 2), or LTB (heat-labile enterotoxin subunit B), are commonly used. We survey papers of successfully secretion of recombinant proteins with specific signal sequences, and choose 3 sequences which were reported to work in the bacterial strains (E. coli DH5α and BL21) we use. They are signal sequences of PhoA [[ #Ref | [6] ]], LTB [[ #Ref | [7] ]], and StII [[ #Ref | [8] ]], named signal peptide 1 (SP1), signal peptide 2 (SP2), and signal peptide 3 (SP3), respectively. Targeting to the Sec pathway, these signal sequences are cleaved in the periplasmic space, which helps to preserve the structural and functional integrity of the original the peptide. This is also an advantage to choose the T2SS as our delivery system. | The selection of signal sequence for delivery of specific recombinant proteins is mostly based on empirical data. Some representative signal sequences, such as those of PhoA (alkaline phosphatase), PelB (pectate lysase B), OmpA (outer-membrane protein A), OmpF (outer-membrane protein F), StII (heat-stable enterotoxin 2), or LTB (heat-labile enterotoxin subunit B), are commonly used. We survey papers of successfully secretion of recombinant proteins with specific signal sequences, and choose 3 sequences which were reported to work in the bacterial strains (E. coli DH5α and BL21) we use. They are signal sequences of PhoA [[ #Ref | [6] ]], LTB [[ #Ref | [7] ]], and StII [[ #Ref | [8] ]], named signal peptide 1 (SP1), signal peptide 2 (SP2), and signal peptide 3 (SP3), respectively. Targeting to the Sec pathway, these signal sequences are cleaved in the periplasmic space, which helps to preserve the structural and functional integrity of the original the peptide. This is also an advantage to choose the T2SS as our delivery system. | ||
| + | [[FIle:NTU-Taida-Project-SP.png|500px|center]] | ||
| + | |||
<html> | <html> | ||
<table class='table table-bordered'> | <table class='table table-bordered'> | ||
Latest revision as of 01:30, 27 October 2012
Effector
We use neuropeptide GLP-1 as the effector. Targeting to the brain, it can modulate the eating behavior of hosts and has neuroprotective function as well. Cell penetrating peptide and signal sequence are designed for facilitation of penetration and secretion, respectively.
Contents |
Glucagon-like Peptide-1
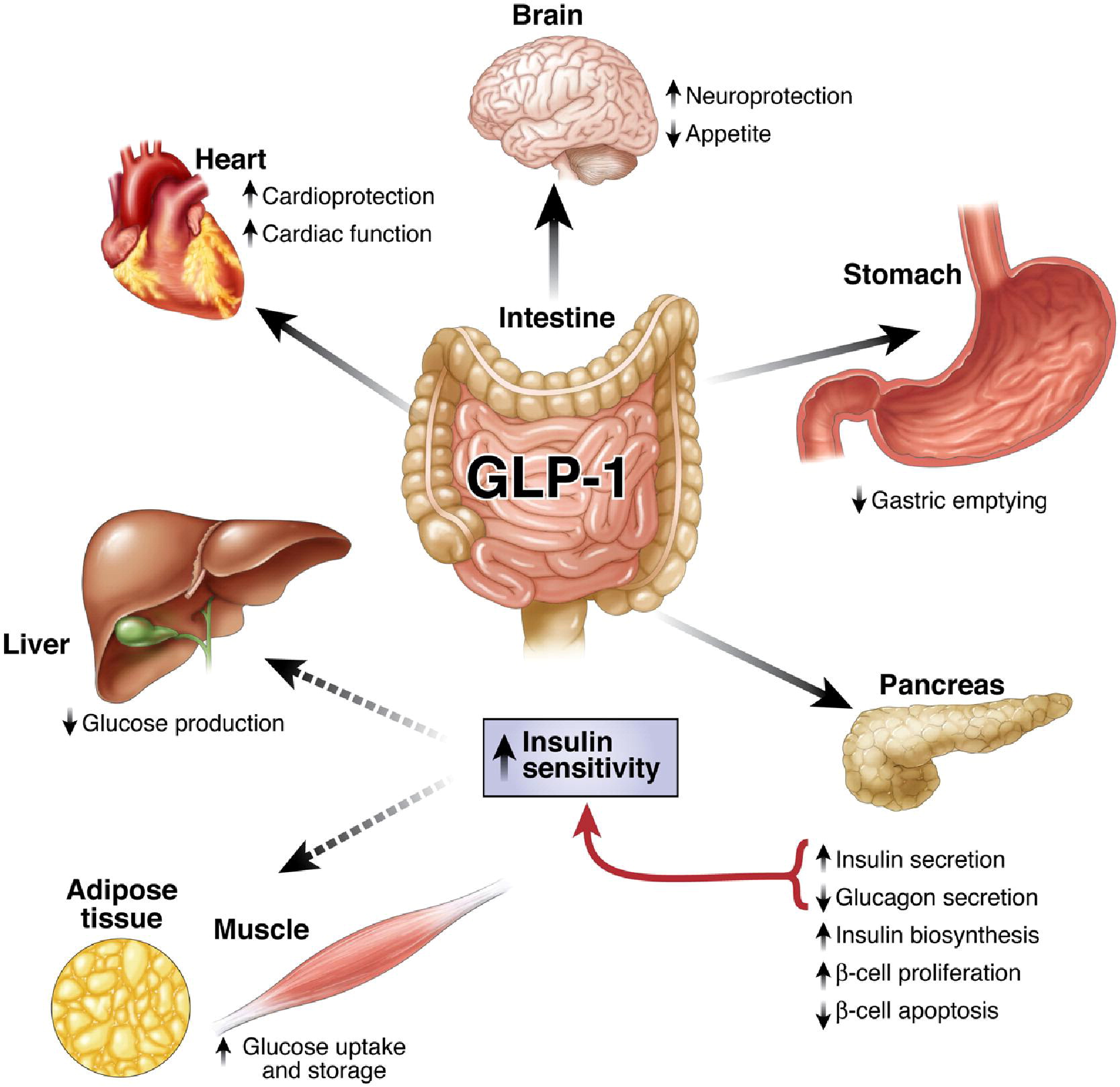
Glucagon-like Peptide-1 (GLP-1) is one of the members of incretin, a group of gastrointestinal hormones secreted by endocrine cells in small intestine that mainly work to increase insulin levels. Under normal physiological condition, this 30-amino acid peptide is secreted by L cells, a kind of neuroendocrine cell located in small intestine after a meal, into the circulation, promoting insulin release and inhibit energy intake. Its natural form can be degraded by DPP-4, having a half life of 1-2 minutes. GLP-1 mainly acts on the GLP-1 receptor, a G-protein coupled receptors, coupled with adenylyl cyclase downstream and signaling on PI3K/Akt and ERK1/2 pathways via second messenger cAMP. It promotes the secretion of insulin in β cells of islets in pancreas, antagonizes and glucose-rising effect of glucagon, and largely increase the insulin sensitivity of targeted cells. GLP-1 analogues such as Liraglutide and Exentide and DPP-4 inhibitors have been used clinically as treatment for patients with type 2 diabetes mellitus (T2DM), but recently, more and more focus has been spotted on its versatile functions, such as cardiovascular regulation, appetite inhibition, and neuroprotective effects. Here we aim to utilize the functions in appetite inhibition and neuroprotection of GLP-1, in order to tailor our PEPDEX as a living machine that communicate with our central nervous systems and modulate our eating behavior, as well as improve our cognitive function to prevent neurodegenerative diseases.
Fat Extinguisher—Reduction in Eating Behavior
Obesity and metabolic syndrome have been regarded as chronic disease recent decades. It is reported that people with obesity or metabolic syndromes are in a higher risk of type II diabetes, cardiovascular disease and hypertension. Nowadays, there are 1 billion people encountering over-weight problem, and 30 million people are diagnosed of obesity. Modern dietary pattern has been considered the major cause contributing to the phenomena. GLP-1 is known to act on the satiety center inside hypothalamic regions of brains, inhibiting appetite and modulating feeding behavior. Therapeutic use of anti-obesity has also been proved by different drug authorities. In PepdEx, we put GLP-1 production under the control of a circuit with a fatty acid sensor, mimicking the physiological response that GLP-1 is released after food intake. Induced by a fat meal, bacterial secreted GLP-1 are expected to penetrate the intestinal epithelium with the assistance of cell penetrating peptide Penetratin, circulating to the brain and perform its behavior-regulating function.
Brain Guardian—Memory Improvement and Neuroprotection
Besides regulation of appetite, GLP-1 also has wide effects on the central nervous system, including promotion of learning and memory and neuroprotective roles. GLP-1 receptors are distributed among the brain in various regions such as hypothalamus, thalamus, brainstem, and hippocampus. Studies using in vitro culture and animal models indicate that GLP-1 promote the learning and memory function in CA1 region of hippocampus, via increase of long-term potentiation (LTP) and enhancement of synaptic plasticity. It also reduces the destructive effects of Aβ amyloid and preserve and dopaminergic neurons in Alzheimer’s disease and Parkinson’s disease, respectively. Potentiation of neuronal differentiation, promotion of cell survival, up-regulation of neurotransmitter synthesis and modulation of glial cells are other functions of GLP-1 in CNS that have been reported, while more diseases such as Huntington’s disease, stroke, and peripheral sensory neuropathy potentially become its therapeutic targets. Conjugation with a thermal regulatory promoter gives a constitutive delivery of GLP-1 inside the human body, which constantly stimulates and protects the CNS, in improvement of learning and memory and prevention of neurodegenerative diseases.
- De Silva A, et al. (2011) The Gut Hormones PYY3-36 and GLP-17-36 amide Reduce Food Intake and Modulate Brain Activity in Appetite Centers in Humans. Cell Metab 14(5):700-6.
- Owais B, et al. (2005) The gastrointestinal tract and the regulation of appetite. Drug Discovery Today: Disease Mechanisms 2(3):289-94.
- MacDonald PE, et al. (2002) The Multiple Actions of GLP-1 on the Process of Glucose-Stimulated Insulin Secretion. Diabetes 51 Suppl 3:S434-42
- Choi S, et al. (2005) Glucagon-like Peptide-1 Plasmid Construction and Delivery for the Treatment of Type 2 Diabetes. Mol Ther 12(5):885-91.
- Baggio LL, et al. (2007) Biology of Incretins: GLP-1 and GIP. Gastroenterology 132(6):2131-57.
- P Puska, et al. Obesity and Overweight. World Health Organization
- Di Marino L, et al. (2011) Active glucagon-like peptide-1 (GLP-1): Storage of human plasma and stability over time. ClinicaChimicaActa 412(17-18):1693-94.
- Degn KB, et al. (2004) One week's treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes 53(5):1187-94.
- Ranganath LR, et al. (1996) Attenuated GLP-1 secretion in obesity: cause or consequence? Gut 38(6):916-9.
- Li Y, et al. (2010) GLP-1 receptor stimulation reduces amyloid-β peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer’s disease. J Alzheimers Dis 19(4): 1205–1219.
- Biswas SC, et al. (2008) Glucagon-like Peptide-1 (GLP-1) Diminishes neuronal degeneration and death caused by NGF deprivation by suppressing Bim induction. Neurochem Res 33:1845–1851.
- Lin L. (2008) Commonality between diabetes and Alzheimer’s disease and a new strategy for the therapy. Clinical Medicine: Pathology 1: 83–91.
- Li Y, et al. (2009) GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Nat Acad Sci 106(4): 1285-1290.
- Mossello E, et al. (2011) Glucagon-like peptide-1, diabetes, and cognitive decline: Possible pathophysiological links and therapeutic opportunities. Experimental Diabetes Research 2011:1-6.
- Abbas T, et al. (2009) Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: Interaction between type 2 diabetes and Alzheimer’s disease. Behavioural Brain Research 205:265–271.
- Holst JJ, et al. (2011) Neuroprotective properties of GLP-1: theoretical and practical applications. Current Medical Research & Opinion 27(3):547–558.
- Bak AM, et al. (2011) Targeting amyloid-beta by glucagon-like peptide -1 (GLP-1) in Alzheimer’s disease and diabetes. Expert Opin. Ther. Targets 15(10):1153-1162.
- McClean PL, et al. (2011) The diabetes drug Liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. The Journal of Neuroscience 31(17):6587–6594.
- Pratley RE. (2008) The new science of GLP-1: Effects beyond glucose control. Adv Stud Med. 8(11):393-399.
Cell Penetrating Peptide
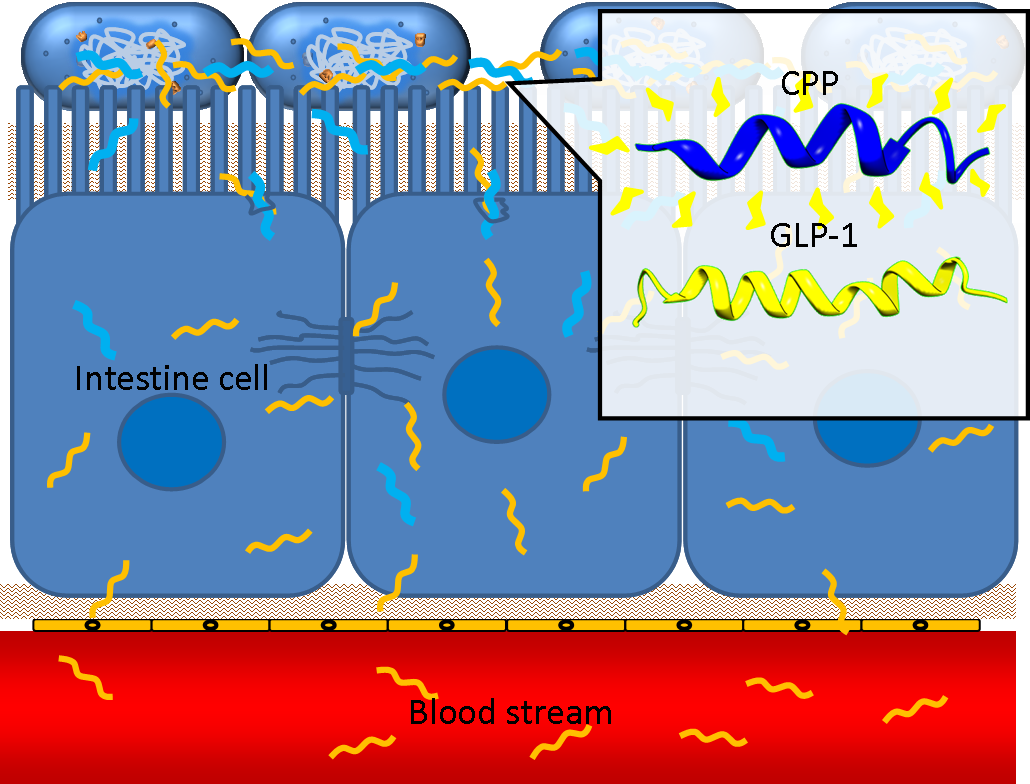
One obstacle needs to be overcome is the poor penetrating ability of GLP-1 through the intestinal epithelia, since GLP-1 is neither small molecule nor hydrophobic. However, several studies have shown that some short peptides known as cell penetrating peptides (CPPs) can help to deliver biodrugs through intestinal mucosa and increase their bioavailability. CPPs are usually arginine-rich, tend to interact with extracellular matrix and can enhance macropinocytosis of epithelial cells. One recent study even demonstrated that co-administration of GLP-1 and Penetratin, one of the CPPs, greatly increased the intestinal absorption rate to 5%. By applying cell penetrating peptide to our device, we aim to promote the delivery of GLP-1 from intestinal lumen to blood stream, therefore enhance their appetite-regulating effect.
| Peptides | Sequence |
| Arginine rich peptides | |
| Arginine octamer (R8) | RRRRRRRR |
| Arginine dodecamer (R12) | RRRRRRRRRRRR |
| HIV-1 Tat (48–60) | GRKKRRQRRRPPQ |
| HIV-1 Rev (34–50) | TRQARRNRRRRWRERQR |
| Amphipathic peptides | |
| Penetratin | RQIKIWFQNRRMKWKK |
| pVEC | LLIILRRRIRKQAHAHSK |
| Erns | RQGAARVTSWLGLQLRIGK |
| RRL helix | RRLRRLLRRLRRLLRRLR |
| PRL4 | PRLPRLPRLPRL |
| Random composition peptides | |
| Random peptide | GLSASPNLQFRTV |
- Khafagy el-S, et al. (2012) Oral biodrug delivery using cell-penetrating peptide. Adv Drug Deliv Rev 64(6):531-9.
- Khafagy el-S, et al. (2009) Efficiency of cell-penetrating peptides on the nasal and intestinal absorption of therapeutic peptides and proteins. Int J Pharm 381(1):49-55.
- Nakase I, et al. (2004) Cellular Uptake of Arginine-Rich Peptides: Roles for Macropinocytosis and Actin Rearrangement. Mol Ther 10(6):1011-22.
Secretion
In order to achieve the extracellular secretion of our recombinant peptide GLP-1 and CPP, we utilize the endogenous type 2 secretion system (T2SS) in bacteria with specific signal peptides added to the N-terminus of GLP-1 and CPP.
Type 2 Secretion System
Secretion systems of gram-negative bacteria are categorized into 6 types, from type 1 secretion system (T1SS) to type 6 secretion system (T6SS). Among these secretion systems, type 1 and type 2 secretion systems are in charge of transport bacterial protein products into the extracellular space. [1] Here we choose type 2 secretion system (T2SS) as our secretion apparatus which is widely found in gram-negative bacteria, including E. coli. Unlike the single-step secretion of T1SS such as hemolysin transport system (Hly), T2SS has two-step secretion processes. Unfolded or folded proteins with specific signal sequences on their N-terminus are first transported through the inner membrane into the periplasmic space via Sec translocon or twin-arginine translocation (Tat) pathway, respectively. [1, 4, 5] The cleavage of signal peptides is performed by signal peptidase inside the periplasm. Proteins of proper structural features are then transported through the outer membrane into the extracellular space via general secretory pathway (GSP) which consists of 4 distinguished subassemblies: inner membrane platform, outer membrane complex, pseudopilus, and secretion ATPase (Fig. 1) [2, 3] , while small proteins (in our case, short peptides) can also leak from the periplasmic into the environment, regarding the integrity of the outer membrane. Co-expressions of some genes, such as kil, out, tolAIII, and bacteriocin release protein (BRP), were reported to facilitate the extracellular secretory processes. [1]
Signal Peptides
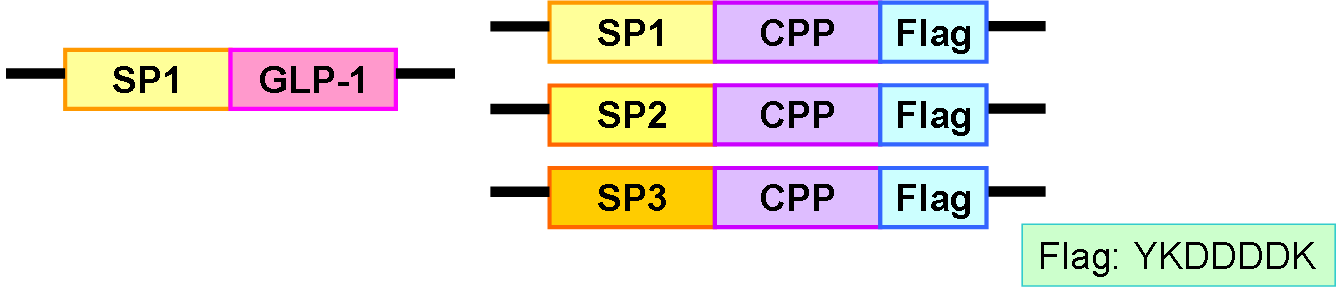
The selection of signal sequence for delivery of specific recombinant proteins is mostly based on empirical data. Some representative signal sequences, such as those of PhoA (alkaline phosphatase), PelB (pectate lysase B), OmpA (outer-membrane protein A), OmpF (outer-membrane protein F), StII (heat-stable enterotoxin 2), or LTB (heat-labile enterotoxin subunit B), are commonly used. We survey papers of successfully secretion of recombinant proteins with specific signal sequences, and choose 3 sequences which were reported to work in the bacterial strains (E. coli DH5α and BL21) we use. They are signal sequences of PhoA [6] , LTB [7] , and StII [8] , named signal peptide 1 (SP1), signal peptide 2 (SP2), and signal peptide 3 (SP3), respectively. Targeting to the Sec pathway, these signal sequences are cleaved in the periplasmic space, which helps to preserve the structural and functional integrity of the original the peptide. This is also an advantage to choose the T2SS as our delivery system.
| Signal Peptide | Source | Sequence |
|---|---|---|
| SP1 | PhoA | MKQSTIALALLPLLFTPVTKA |
| SP2 | LTB | MNKVKCYVLFTALLSSLYAHG |
| SP3 | StII | MKKNIAFLLASMFVFSIATNAYA |
Reference
- Choi JH, et al. (2004) Secretory and extracellular production of recombinant proteins using Escherichia coli. Appl Microbiol Biotechnol 64: 625-635.
- Korotkov KV, et al. (2012) The type II secretion system: biogenesis, molecular architecture and mechanism. Nat Rev Microbiol 10(5):336-51.
- Filloux A (2004) The underlying mechanisms of type II protein secretion. Biochim Biophys Acta 1694(1-3):163-79.
- Muller M, et al. (2005) The Tat pathway in bacteria and chloroplasts. Molecular Membrane Biology 22(1-2): 113-121.
- 5DeLisa MP, et al. (2003) Folding quality control in the export of proteins by the bacterial twin-arginine translocation pathway. Proc Natl Acad Sci 100(10):6115-20.
- Xu R, et al. (2002) High-level expression and secretion of recombinant mouse endostatin by Escherichia coli. Protein Expr Purif 24:453-459.
- Pillai D, et al. (1996) Production of the beta-subunit of human chorionic gonadotropin in Escherichia coli and its export mediated by the heat-labile enterotoxin chain-B signal sequence. Gene 173(2):271-274.
- Dracheva S, et al. (1995) Expression of soluble human interleukin-2 receptor α-chain in Escherichia coli. Protein Expr Purif 6:737-747.
- Mitra K, et al. (2005) Structure of E. coli protein-conducting channel bound to a translating ribosome. Nature 438:318-24.
- Dekker C, et al. (2003) Crystal structure of SecB from Escherichia coli. Journal of Structural Biology 144:313-319.
- Mergulhao FJM, et al. (2005) Recombinant protein secretion in Escherichia coli. Biotechnology Advances 23:177-202.
- Papanikolau Y, et al. (2006) Structure of Dimeric SecA, the Escherichia coli Preprotein Translocase Motor. J Mol Biol 366:1545-1557.
- Steiner D, et al. (2006) Signal sequences directing cotranslational translocation expand the range of proteins amenable to phage display. Nature Biotechnology 24:823-31.
 "
"