Team:Bielefeld-Germany/Project/Background
From 2012.igem.org
KevinJarosch (Talk | contribs) |
|||
| (One intermediate revision not shown) | |||
| Line 145: | Line 145: | ||
__NOTOC__ | __NOTOC__ | ||
| - | + | Due to the remarkable negative effects on the environment the following substrates were chosen to investigate the degradation potential of our produced laccases. These substrates represent typical micro contaminants in the natural water body. | |
| Line 168: | Line 168: | ||
==Ethinyl estradiol:== | ==Ethinyl estradiol:== | ||
[[File:Bielefeld2012-ethinylestradiol.png|thumb|Figure 3: Structure of ethinyl estradiol. [http://www.tcichemicals.com/eshop/en/eu/commodity/E0037/ Source of the figure]]] | [[File:Bielefeld2012-ethinylestradiol.png|thumb|Figure 3: Structure of ethinyl estradiol. [http://www.tcichemicals.com/eshop/en/eu/commodity/E0037/ Source of the figure]]] | ||
| - | Ethinyl estradiol is the synthetic variation of the natural estradiol. | + | Ethinyl estradiol is the synthetic variation of the natural estradiol. Due to it's structure it is not easily degradable in humans. Birth control pills contain ethinyl estradiol that passes the body via excretion into wastewater which reaches sewage plants and from there lake water where fishes and other organisms live. This causes several disorders in the natural metabolisms of the organisms such as the feminization of fish.<sup>[3]</sup> The increase of ethinyl estradiol in wastewater in the past years has made ethinyl estradiol to a potential problem in the future. But not only fish also humans are affected from this problem because the wastewater from sewage seeps into groundwater and from there into drinking water. Therefore the degradation or filtration of ethinyl estradiol will become more important in cleaning water. Activated carbon or ozonation treatment of wastewater could solve this problem but it is too expensive in practice. Such an activated carbon treatment in England would cost around $10,3 million for 250.000 people.<sup>[4]</sup> <br> |
UV-light detection: 230 nm Extinction, 310 nm Emission <br> | UV-light detection: 230 nm Extinction, 310 nm Emission <br> | ||
| Line 176: | Line 176: | ||
==Diclofenac:== | ==Diclofenac:== | ||
[[File:Bielefeld2012-diclofenac.png|thumb|Figure 4: Structure of diclofenac. [https://www.caymanchem.com/app/template/Product.vm/catalog/70680 Source of the figure]]] | [[File:Bielefeld2012-diclofenac.png|thumb|Figure 4: Structure of diclofenac. [https://www.caymanchem.com/app/template/Product.vm/catalog/70680 Source of the figure]]] | ||
| - | Diclofenac is used to reduce inflammation. As an analgesic it reduces pain in certain conditions and reaches through wastewater lakes and drinking water. Since this chemical, like ethinyl estradiol is not filterable in sewages without activated carbon or ozonation treatment.<sup>[5]</sup> | + | Diclofenac is used to reduce inflammation. As an analgesic it reduces pain in certain conditions and reaches through wastewater lakes and drinking water. Since this chemical, like ethinyl estradiol is not filterable in sewages without activated carbon or ozonation treatment.<sup>[5]</sup> Diclofenac and ethinyl estradiol are getting [http://europa.eu/rapid/pressReleasesAction.do?reference=IP/12/88&format=HTML&aged=0&language=EN&guiLanguage=en more attention] from the [http://ec.europa.eu/environment/water/index_en.htm European Commision for Water]. Besides there is a precise example in the [http://www.schlossholtestukenbrock.de/060/sr_seiten/artikel/112120100000015850.php sewage plant Schloß-Holte], where a concentration of 1900 ng/L diclofenac was detected. For more information about the risks read the [http://www.umweltbundesamt.de/chemikalien/veranstaltungen/ws-monitoring-arzneimittel/3_vortrag-abstract_vietoris.pdf GOW]. |
Molecular Mass: 296,148 g * mol<sup>-1</sup> <br> | Molecular Mass: 296,148 g * mol<sup>-1</sup> <br> | ||
Chemical structure:C<sub>14</sub>H<sub>10</sub>Cl<sub>2</sub>NO<sub>2</sub>Na | Chemical structure:C<sub>14</sub>H<sub>10</sub>Cl<sub>2</sub>NO<sub>2</sub>Na | ||
| Line 182: | Line 182: | ||
==Ibuprofen:== | ==Ibuprofen:== | ||
[[File:Bielefeld2012-ibuprofen.png|thumb|Figure 5: Structure of ibuprofen. [https://www.caymanchem.com/app/template/Product.vm/catalog/70280 Source of the figure]]] | [[File:Bielefeld2012-ibuprofen.png|thumb|Figure 5: Structure of ibuprofen. [https://www.caymanchem.com/app/template/Product.vm/catalog/70280 Source of the figure]]] | ||
| - | Ibuprofen is as well as diclofenac used in pain treatments like in the case of rheumatism. The World Health Organization ([http://www.who.int/en/ WHO]) | + | Ibuprofen is as well as diclofenac used in pain treatments like in the case of rheumatism. The World Health Organization ([http://www.who.int/en/ WHO]) set ibuprofen on the [http://www.who.int/medicines/publications/essentialmedicines/Updated_sixteenth_adult_list_en.pdf list of essential medicine]. According to a medicine study, people who take regularly ibuprofen have a higher risk of getting kidney cancer.<sup>[6]</sup> <br> |
UV- light detection: Extinction 224nm, Emission 290nm <br> | UV- light detection: Extinction 224nm, Emission 290nm <br> | ||
| Line 190: | Line 190: | ||
==Naproxen:== | ==Naproxen:== | ||
[[File:Bielefeld2012-naproxen.png|thumb|Figure 6: Structure of naproxen. [https://www.caymanchem.com/app/template/Product.vm/catalog/70290 Source of the figure]. ]] | [[File:Bielefeld2012-naproxen.png|thumb|Figure 6: Structure of naproxen. [https://www.caymanchem.com/app/template/Product.vm/catalog/70290 Source of the figure]. ]] | ||
| - | Naproxen is also used in pain treatment. Besides its helpful | + | Naproxen is also used in pain treatment. Besides its helpful properties, naproxen can also cause several side effects such as hoarseness or nausea.<sup>[7]</sup> Naproxen reaches natural water through the wastewater. <br> |
| - | UV- light detection: Extinction | + | UV- light detection: Extinction 232 nm, Emission 272 nm <br> |
Molecular Mass: 230,3 g * mol<sup>-1</sup> <br> | Molecular Mass: 230,3 g * mol<sup>-1</sup> <br> | ||
Chemical structure: C<sub>14</sub>H<sub>14</sub>O<sub>3 <br><br><br><br> | Chemical structure: C<sub>14</sub>H<sub>14</sub>O<sub>3 <br><br><br><br> | ||
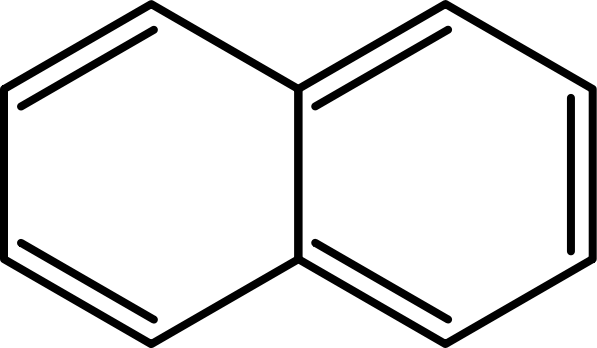
==Naphthalene:== | ==Naphthalene:== | ||
| - | [[File:Bielefeld2012-597px-Naphthalene-2D-Skeletal.png|thumb|Figure 7: Structure of | + | [[File:Bielefeld2012-597px-Naphthalene-2D-Skeletal.png|thumb|Figure 7: Structure of naphthalene. [http://en.wikipedia.org/wiki/File:Naphthalene-2D-Skeletal.svg Source of the figure]]] |
| - | + | Naphtalene is commonly used as an intermediate for some chemical synthesis of insect repellents or fungicides in the industry. From industrial use naphthalenes reaches sewage water and from there lake. Naphtalene may destroy red blood cells when overdosed in blood. This would be fatal for fishes in lakes. It is already shown that the laccase from ''E. coli'' which we produced as well is able to degrade naphtalene.<sup>[8]</sup> That is why we used naphtalene to test the activity of the expressed laccases. <br> | |
UV-light detection: Extinction 275 nm, Emission 350 nm <br> | UV-light detection: Extinction 275 nm, Emission 350 nm <br> | ||
| Line 205: | Line 205: | ||
==Acenaphthene:== | ==Acenaphthene:== | ||
| - | [[File:Bielefeld2012-107px-Acenaphthene.png|thumb|Figure 8: Structure of | + | [[File:Bielefeld2012-107px-Acenaphthene.png|thumb|Figure 8: Structure of acenaphtene. [http://de.wikipedia.org/w/index.php?title=Datei:Acenaphthene.svg&filetimestamp=20070113040401 Source of the figure]]] |
| - | This chemical is a polycyclic aromatic hydrocarbon (PAH) consisting of naphthalene with an ethylene bridge and is used in the preparation of pesticides and pharmaceuticals. It reaches the ground water the same way as the other chemicals. The effects on organisms are not yet clearly defined. But the ''E.coli'' Cue0 laccase is able to | + | This chemical is a polycyclic aromatic hydrocarbon (PAH) consisting of naphthalene with an ethylene bridge and is used in the preparation of pesticides and pharmaceuticals. It reaches the ground water the same way as the other chemicals. The effects on organisms are not yet clearly defined. But the ''E.coli'' Cue0 laccase is able to degrade acenaphtene.<sup>[8]</sup> <br> |
| - | UV-light detection: Extinction | + | UV-light detection: Extinction 275 nm, Emission 350 nm <br> |
Molecular Mass: 154,21g * mol<sup>-1</sup> <br> | Molecular Mass: 154,21g * mol<sup>-1</sup> <br> | ||
Chemical structure: C<sub>12</sub>H<sub>10</sub> <br><br><br><br> | Chemical structure: C<sub>12</sub>H<sub>10</sub> <br><br><br><br> | ||
| Line 216: | Line 216: | ||
Its name comes from phenyl and anthracene. Phenanthere is used in some explosives and drugs. There is not much information about the effects or interactions in water or with organisms in water but the ''E.coli'' laccase CueO was able to degrade this chemical.<sup>[8]</sup> <br> | Its name comes from phenyl and anthracene. Phenanthere is used in some explosives and drugs. There is not much information about the effects or interactions in water or with organisms in water but the ''E.coli'' laccase CueO was able to degrade this chemical.<sup>[8]</sup> <br> | ||
| - | UV-light detection: Extinction | + | UV-light detection: Extinction 275 nm, Emission 350 nm <br> |
| - | Molecular Mass: 178, | + | Molecular Mass: 178,23 g * mol<sup>-1</sup> <br> |
Chemical structure: C<sub>14</sub>H<sub>10</sub> <br><br><br><br><br> | Chemical structure: C<sub>14</sub>H<sub>10</sub> <br><br><br><br><br> | ||
==Anthracene:== | ==Anthracene:== | ||
| - | [[File:Bielefeld2012-800px-Anthracene-2D-Skeletal.png|thumb|Figure 10: Structure of | + | [[File:Bielefeld2012-800px-Anthracene-2D-Skeletal.png|thumb|Figure 10: Structure of anhtracene. [http://de.wikipedia.org/w/index.php?title=Datei:Anthracene_structure.png&filetimestamp=20070610032248 Source of the figure]]] |
| - | Anthracene is used in producing dyes but the consequences for human or other organisms are not yet cleared. Because the ''E.coli'' laccase CueO was able to degrade other polycyclic aromatic hydrocarbons we decide to test | + | Anthracene is used in producing dyes but the consequences for human or other organisms are not yet cleared. Because the ''E.coli'' laccase CueO was able to degrade other polycyclic aromatic hydrocarbons we decide to test anthracene with the laccases we produced. <br> |
| - | UV-light detection: Extinction | + | UV-light detection: Extinction 260 nm, Emission 430 nm <br> |
| - | Molecular Mass: 178, | + | Molecular Mass: 178,24 g * mol<sup>-1</sup> <br> |
Chemical structure: C<sub>14</sub>H<sub>10</sub> <br><br><br><br> | Chemical structure: C<sub>14</sub>H<sub>10</sub> <br><br><br><br> | ||
| Line 256: | Line 256: | ||
</html> | </html> | ||
| - | In the last few years a lot of attention has been drawn to Laccases due to their ability to | + | In the last few years a lot of attention has been drawn to Laccases due to their ability to oxidize both phenolic and nonphenolic lignin related compounds as well as highly recalcitrant environmental pollutants. This makes them very useful for applications concerning several biotechnological processes. This includes the detoxification of industrial effluents, for example from the paper and pulp, textile and petrochemical industries. Laccases are also valuable as a tool as a tool for medical diagnostics and as a bioremediation agent to clean up herbicides, pesticides and certain explosives in soil. Furthermore these enzymes are also used as catalysts for the manufacture of anti-cancer drugs and even as ingredients in cosmetics<sup>[1]</sup>. |
Their capacity to remove xenobiotic substances and produce polymeric products makes them a useful tool for bioremediation purposes. In our project laccases are used as cleaning agents for a water purification system. | Their capacity to remove xenobiotic substances and produce polymeric products makes them a useful tool for bioremediation purposes. In our project laccases are used as cleaning agents for a water purification system. | ||
| - | Laccases are copper-containing polyphenol | + | Laccases are copper-containing polyphenol oxidaze enzymes '''(EC 1.10.3.2)''' that can be found in many plants, insects, microorganisms and mainly in fungi. These enzymes fulfill several functions in different metabolic pathways. One the one hand these enzymes are necessary to degrade lignin in ''Basidiomycetes'' and on the other hand to synthesize complex polymers like Melanin in ''Ascomycentes''. In general, laccases are extracellular enzymes and consists usually of 15-20 % carbon-hydrogen. The molecular weight of the deglycated protein ranges from 60 to 80 kDa. These enzymes can occur as monomers, dimers, trimers and tetramers. The first crystal structure of a laccase from the organism ''Trametes versicolor'' was published in 2002 <sup>[2]</sup>. The structure is illustrated in the video at the end of this text. |
Laccases are able to oxidize a broad range of substrates due to the contained copper-cluster, by reducing oxygen to water. The active site of the enzyme includes a four-copper-ion-cluster, which can be distinguished by spectroscopic analyses. This cluster consists of one blue copper-ion (type 1), one type 2 and two type 3 copper-ions. Because of the blue copper-ion, the laccases belong to the big family of the blue copper proteins. This specific blue copper ion is essential for the enzyme mediated radical oxidation of the phenolic groups. In this reaction the electron from the oxidation is transferred to the other three copper ions. These ions form a trinuclearic cluster, which transfers electrons to the terminal electron acceptor oxygen. By receiving four electrons the molecular oxygen is finally reduced to water. | Laccases are able to oxidize a broad range of substrates due to the contained copper-cluster, by reducing oxygen to water. The active site of the enzyme includes a four-copper-ion-cluster, which can be distinguished by spectroscopic analyses. This cluster consists of one blue copper-ion (type 1), one type 2 and two type 3 copper-ions. Because of the blue copper-ion, the laccases belong to the big family of the blue copper proteins. This specific blue copper ion is essential for the enzyme mediated radical oxidation of the phenolic groups. In this reaction the electron from the oxidation is transferred to the other three copper ions. These ions form a trinuclearic cluster, which transfers electrons to the terminal electron acceptor oxygen. By receiving four electrons the molecular oxygen is finally reduced to water. | ||
====Structure of the Laccase from ''Trametes versicolor''==== | ====Structure of the Laccase from ''Trametes versicolor''==== | ||
| - | The | + | The video was done with the [http://jmol.sourceforge.net/ Jmol-software] in combination with the data from the [http://www.rcsb.org/pdb/explore/explore.do?structureId=1GYC RCSB Protein Data Bank] |
<html> | <html> | ||
Latest revision as of 00:21, 27 October 2012
Chemical waste in water?!
Water is the source of all life and it covers 71 % of the earth’s surface. How could its importance be more highlighted than by its nickname "the blue planet"? Healthy drinking water is an important aspect and essential for mankind. However, the growing industrialization, the production of chemical agents and the increasing consumption of pharmaceuticals are some of the causes of rapidly increasing pressure on the aquatic environment as well as on the availability and quality of safe and clean water. The continuous release of pharmaceuticals into the environment and their negative effects on biological systems are not proven. The measured environmental concentrations are responsible for the fact that water pollution is one of the main environmental worries of our society. According to a survey of March 2012 from the Public Opinion Analysis sector of the [http://www.europarl.europa.eu/meetdocs/2009_2014/documents/envi/pr/909/909091/909091en.pdf European Commission],
- 68 % of Europeans think that water quantity and quality problems are serious.
- 80 % believe that chemical pollution is a threat to the water environment.
- 62 % feel that they are not sufficiently informed about problems facing groundwater, lakes, rivers and coastal waters in their countries.
The birth control pill is a widespread contraception method. However, large amounts of the active compounds, i.e. modified estrogens, leave the body via the urine. According to the Federal Environment Agency in Germany (Umweltbundesamt, UBA) several hundred tons of analgesics, antibiotics, beta blockers, X-ray contrast agents, anti-epileptic drugs, polyaromatic hydrocarbons pesticides etc. enter the waste water and end up in rivers, lakes and consequently in drinking water.
The most human-derived substances which can be detected in the surface water possess at least one aromatic ring structure. Due to the aromatic structures, these substances are not effectively degraded in the sewage treatment plants with conventional methods. This means that a high proportion of these substances is being released into the environment. According to the Rhine Westphalian Institute of Water Research gGmbH (IWW) environmental concentrations of [http://www.umweltbundesamt.de/chemikalien/veranstaltungen/ws-monitoring-arzneimittel/11_vortrag-abstract_bergmann.pdf 247] human and veterinary drugs can be detected in the environmental sewage effluent, the surface water, the groundwater, the drinking water and the sewage sludge. These substances include:
Sweeteners: Acesulfam, Sucralose
Antibiotics: Clarithromycin, Sulfamethoxazol, N4-Acetylsulfamethoxazol , Carbamazepin
Analgetics: Diclofenac, Ibuprofen
Benzotriazole: Benzotriazol, 4-Methylbenzotriazol, 5-Methylbenzotriazol,
Beta-Blocker: Metoprolol, Sotalol
X-Ray contrast agents: Amidotrizoeacid, Iomeprol, Iopamidol, Iopromid
Subsequently to the wastewater treatment, the concentrations of these substances and their corresponding metabolites are below their therapeutically effective concentration so that these agents are classified as concepts micro-contaminants. But the impacts of the micro-contaminants on the environment are already evident. For example [http://toxsci.oxfordjournals.org/content/106/1/93.short Shved et al. 2008] has shown that 17a-Ethinylestradiol in an environmentally relevant concentration is able to influence fish growth and reproductive functions of bony fishes.
The long-term consequences of an increasing estrogen concentration for human beings are still largely unknown. Nonetheless, declining sperm counts and thereby increasing infertility in men living in industrial nations may well relate to this hormonal pollution. In addition, [http://bmjopen.bmj.com/content/1/2/e000311.full testicular and prostate cancers] as well as osteoporosis could be a consequence of overly high concentrations of estrogen in the human body. At the moment only the acute and short term effects are obvious, but the long term effects of continuous exposure of micro-contaminates to ecosystems and the effects that occur even below therapeutic levels in non-target organisms are not [http://www.umweltbundesamt.de/chemikalien/veranstaltungen/ws-monitoring-arzneimittel/16_vortrag-abstract_knacker.pdf predictable].
Only for 70 of the [http://www.umweltbundesamt.de/chemikalien/veranstaltungen/ws-monitoring-arzneimittel/11_vortrag-abstract_bergmann.pdf 247] substances sufficient information exist for an ecotoxicological assessment. To assess the risk, the Federal Environment Agency (UBA) published a recommendation for not or only partially assessable micro-contaminants, defined as “risk for your healthy value" [http://www.umweltbundesamt.de/chemikalien/veranstaltungen/ws-monitoring-arzneimittel/3_vortrag-abstract_vietoris.pdf GOW]. Some of these recommended values already have been exceeded by several agents, like Diclofenac (up to 10,5µg L-1) and Ibuprofen up to 9,79 µg L-1).
Detailed concentrations in sewage effluent and surface water of Diclofenac and some other substances can be found here. The increasing amounts of some micro-contaminants, prompted the European Commission to add [http://www.europarl.europa.eu/meetdocs/2009_2014/documents/envi/pr/909/909091/909091en.pdf 15 new priority substances] to the list of priority hazardous substances:
- six ingredients of pesticides (Aclonifen, bifenox, cypermethrin, dicofol, heptachlor and quinoxyfen),
- six ingredients of biocides (Dichlorvos and terbutryn),
- two industrial chemicals (perfluoroctansulfonate (PFOS) and hexabromcyclododecan (HBCDD)),
- three pharmaceutical agents (diclofenac, 17a-ethinylestradiol (EE2) und estradiol (E2)) and
- Dioxin and dioxin like polychlorinated biphenyls (dl-PCB).
This list defines priority substances in the field of water policy, namely chemicals presenting a significant risk to or via the aquatic environment. Beside these 15 substances the European commission plans to [http://www.europarl.europa.eu/meetdocs/2009_2014/documents/envi/pr/909/909091/909091en.pdf increase the priorization of other substances] such as anthracene, brominated diphenylether, naphthalene and polycyclic aromatic hydrocarbons.
Nonetheless, there are currently no legally binding limits for concentrations of pharmaceutically active compounds in surface water, groundwater or drinking water. Consequently the increasing consumption of pharmaceuticals lead to an increasing pressure on the aquatic environment and on the availability and quality of healthy and clean water. This is the problem the our iGEM team wants to take care of. The idea of the Bielefeld iGEM team is to developing a biological filter using immobilized laccases to purify municipal and industrial wastewater from synthetic estrogens and other micro-contaminants.
Our Focus
Due to the remarkable negative effects on the environment the following substrates were chosen to investigate the degradation potential of our produced laccases. These substrates represent typical micro contaminants in the natural water body.
Estradiol:

Estradiol is a natural human hormone secreted not only in women but also in men. Its importance in women metabolism is that after reaching puberty this hormone provides being a woman. In men on the other hand estradiol has been detected to have influence on sperm ejaculation.[1] This hormone has particularly effect on female characteristics for example in breast development, changing body shapes or texture of bones. During the menstrual cycle, estradiol levels fluctuates indicating its importance in sexual development.[2]
Since estradiol is easily degradable by many sorts of enzymes we thought it would be a good substrate to start from. Its structural similarity to the hardly to degradable synthetic Ethinyl estradiol could make it a good indicator for higher potential.
UV-light detection: 230 nm Extinction, 310 nm Emission
Molecular Mass: 272,39 g * mol-1
Chemical structure: C18H24O2
Estrone:

Estrone is one of several natural estrogens. Like estradiol it is important in several female development steps. It has been said that estrone disordered people has a higher potential for breast cancer ([http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@rn+53-16-7 HSDB]). Our focus is to characterize the activity of the laccases regarding several substrates similar to estradiol like estrone.
UV-light detection: 230nm Extinction, 310nm Emission
Molecular Mass: 270,36g * mol -1
Chemical structure: C18H22O2
Ethinyl estradiol:
Ethinyl estradiol is the synthetic variation of the natural estradiol. Due to it's structure it is not easily degradable in humans. Birth control pills contain ethinyl estradiol that passes the body via excretion into wastewater which reaches sewage plants and from there lake water where fishes and other organisms live. This causes several disorders in the natural metabolisms of the organisms such as the feminization of fish.[3] The increase of ethinyl estradiol in wastewater in the past years has made ethinyl estradiol to a potential problem in the future. But not only fish also humans are affected from this problem because the wastewater from sewage seeps into groundwater and from there into drinking water. Therefore the degradation or filtration of ethinyl estradiol will become more important in cleaning water. Activated carbon or ozonation treatment of wastewater could solve this problem but it is too expensive in practice. Such an activated carbon treatment in England would cost around $10,3 million for 250.000 people.[4]
UV-light detection: 230 nm Extinction, 310 nm Emission
Molecular Mass: 296,4 g * mol-1
Chemical structure: C20H24O2
Diclofenac:

Diclofenac is used to reduce inflammation. As an analgesic it reduces pain in certain conditions and reaches through wastewater lakes and drinking water. Since this chemical, like ethinyl estradiol is not filterable in sewages without activated carbon or ozonation treatment.[5] Diclofenac and ethinyl estradiol are getting [http://europa.eu/rapid/pressReleasesAction.do?reference=IP/12/88&format=HTML&aged=0&language=EN&guiLanguage=en more attention] from the [http://ec.europa.eu/environment/water/index_en.htm European Commision for Water]. Besides there is a precise example in the [http://www.schlossholtestukenbrock.de/060/sr_seiten/artikel/112120100000015850.php sewage plant Schloß-Holte], where a concentration of 1900 ng/L diclofenac was detected. For more information about the risks read the [http://www.umweltbundesamt.de/chemikalien/veranstaltungen/ws-monitoring-arzneimittel/3_vortrag-abstract_vietoris.pdf GOW].
Molecular Mass: 296,148 g * mol-1
Chemical structure:C14H10Cl2NO2Na
Ibuprofen:

Ibuprofen is as well as diclofenac used in pain treatments like in the case of rheumatism. The World Health Organization ([http://www.who.int/en/ WHO]) set ibuprofen on the [http://www.who.int/medicines/publications/essentialmedicines/Updated_sixteenth_adult_list_en.pdf list of essential medicine]. According to a medicine study, people who take regularly ibuprofen have a higher risk of getting kidney cancer.[6]
UV- light detection: Extinction 224nm, Emission 290nm
Molecular Mass: 206,3g * mol-1
Chemical structure: C13H18O2
Naproxen:
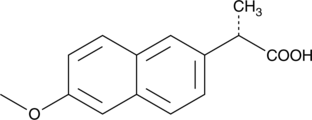
Naproxen is also used in pain treatment. Besides its helpful properties, naproxen can also cause several side effects such as hoarseness or nausea.[7] Naproxen reaches natural water through the wastewater.
UV- light detection: Extinction 232 nm, Emission 272 nm
Molecular Mass: 230,3 g * mol-1
Chemical structure: C14H14O3
Naphthalene:
Naphtalene is commonly used as an intermediate for some chemical synthesis of insect repellents or fungicides in the industry. From industrial use naphthalenes reaches sewage water and from there lake. Naphtalene may destroy red blood cells when overdosed in blood. This would be fatal for fishes in lakes. It is already shown that the laccase from E. coli which we produced as well is able to degrade naphtalene.[8] That is why we used naphtalene to test the activity of the expressed laccases.
UV-light detection: Extinction 275 nm, Emission 350 nm
Molecular Mass: 128,17 g*mol-1
Chemical structure: C10H8
Acenaphthene:
This chemical is a polycyclic aromatic hydrocarbon (PAH) consisting of naphthalene with an ethylene bridge and is used in the preparation of pesticides and pharmaceuticals. It reaches the ground water the same way as the other chemicals. The effects on organisms are not yet clearly defined. But the E.coli Cue0 laccase is able to degrade acenaphtene.[8]
UV-light detection: Extinction 275 nm, Emission 350 nm
Molecular Mass: 154,21g * mol-1
Chemical structure: C12H10
Phenanthrene:
Its name comes from phenyl and anthracene. Phenanthere is used in some explosives and drugs. There is not much information about the effects or interactions in water or with organisms in water but the E.coli laccase CueO was able to degrade this chemical.[8]
UV-light detection: Extinction 275 nm, Emission 350 nm
Molecular Mass: 178,23 g * mol-1
Chemical structure: C14H10
Anthracene:
Anthracene is used in producing dyes but the consequences for human or other organisms are not yet cleared. Because the E.coli laccase CueO was able to degrade other polycyclic aromatic hydrocarbons we decide to test anthracene with the laccases we produced.
UV-light detection: Extinction 260 nm, Emission 430 nm
Molecular Mass: 178,24 g * mol-1
Chemical structure: C14H10
References:
[1] Sharpe RM, Skakkebaek NE (1993). "Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract?" The Lancet 341(8857):1392-1395.
[2] Sonya S. Dasharathy et al. (2011). "Menstrual Bleeding Patterns Among Regularly Menstruating Women." American Journal of Epidemiology 175(6):536-545.
[3] Kidd KA et al. (2007). "Collapse of a fish population after exposure to a synthetic estrogen PNAS 104(21):8897-8901.
[4] Owen R, Jobling S (2012). "Environmental science: The hidden costs of flexible fertility." Nature 485(7399):441
[5] Beltrán FJ et al. (2009). "Diclofenac remocal from water with ozone and activated carbon." Journal of Hazardous Materials 163(2-3):768-776.
[6] Bloomberg, http://www.bloomberg.com/news/2011-09-12/commonly-used-pain-pills-increase-kidney-cancer-risk-in-study.html on www.bloomberg.com Sept. 13, 2012
[7] PubMed Health, http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0000526/ on http://www.ncbi.nlm.nih.gov/ Sept. 14, 2012
[8] Zeng J, Lin X et al. (2011). "Oxidation of polycyclic aromatic hydrocarbons by the bacterial laccase CueO from E. coli." Appl Microbiol Biotechnol 89(6): 1841-1849.
Laccase - Our Teammate
In the last few years a lot of attention has been drawn to Laccases due to their ability to oxidize both phenolic and nonphenolic lignin related compounds as well as highly recalcitrant environmental pollutants. This makes them very useful for applications concerning several biotechnological processes. This includes the detoxification of industrial effluents, for example from the paper and pulp, textile and petrochemical industries. Laccases are also valuable as a tool as a tool for medical diagnostics and as a bioremediation agent to clean up herbicides, pesticides and certain explosives in soil. Furthermore these enzymes are also used as catalysts for the manufacture of anti-cancer drugs and even as ingredients in cosmetics[1]. Their capacity to remove xenobiotic substances and produce polymeric products makes them a useful tool for bioremediation purposes. In our project laccases are used as cleaning agents for a water purification system.
Laccases are copper-containing polyphenol oxidaze enzymes (EC 1.10.3.2) that can be found in many plants, insects, microorganisms and mainly in fungi. These enzymes fulfill several functions in different metabolic pathways. One the one hand these enzymes are necessary to degrade lignin in Basidiomycetes and on the other hand to synthesize complex polymers like Melanin in Ascomycentes. In general, laccases are extracellular enzymes and consists usually of 15-20 % carbon-hydrogen. The molecular weight of the deglycated protein ranges from 60 to 80 kDa. These enzymes can occur as monomers, dimers, trimers and tetramers. The first crystal structure of a laccase from the organism Trametes versicolor was published in 2002 [2]. The structure is illustrated in the video at the end of this text.
Laccases are able to oxidize a broad range of substrates due to the contained copper-cluster, by reducing oxygen to water. The active site of the enzyme includes a four-copper-ion-cluster, which can be distinguished by spectroscopic analyses. This cluster consists of one blue copper-ion (type 1), one type 2 and two type 3 copper-ions. Because of the blue copper-ion, the laccases belong to the big family of the blue copper proteins. This specific blue copper ion is essential for the enzyme mediated radical oxidation of the phenolic groups. In this reaction the electron from the oxidation is transferred to the other three copper ions. These ions form a trinuclearic cluster, which transfers electrons to the terminal electron acceptor oxygen. By receiving four electrons the molecular oxygen is finally reduced to water.
Structure of the Laccase from Trametes versicolor
The video was done with the [http://jmol.sourceforge.net/ Jmol-software] in combination with the data from the [http://www.rcsb.org/pdb/explore/explore.do?structureId=1GYC RCSB Protein Data Bank]
References
[1] Susana Rodríguez Couto & José Luis Toca Herrera;Industrial and biotechnological applications of laccases: A review; 2006; Biotechnology Advances 24 500–513
[2] O. V. Morozova, G. P. Shumakovich, M. A. Gorbacheva, S. V. Shleev, and A. I. Yaropolov, “Blue” Laccases ,Biokhimiya, 2007, Vol. 72, No. 10, pp. 1396-1412.
Laccase-donators
Arabidopsis thaliana
Arabidopsis thaliana (A. thaliana), or commonly known as thale cress, is a small flowering plant which is an established model organism. We have chosen this little plant as representative for higher eukaryotes and not only because there is a lot of research going on at Bielefeld University with this organism. A. thaliana has a small genome with 125 Mb total and was sequenced completely in year 2000. Besides the sophisticated annotation of genes there is also a large number of mutant lines available. The research on A. thaliana is progressing fast and researchers from all around the world are working with this model organism because of its rapid life cycle of six weeks and its simple growth conditions, just to name a few examples. The Arabidopsis community is therefore huge and progress in understanding the cellular and molecular biology of this flowering plant is documented well and available.
For our project we were interested in the laccase(s) of A. thaliana. We did some research at [http://www.arabidopsis.org/servlets/Search?type=general&search_action=detail&method=1&show_obsolete=F&name=laccase&sub_type=gene&SEARCH_EXACT=4&SEARCH_CONTAINS=1/ TAIR][1] and found 22 laccases or laccase-like genes. Stunned by the amount of laccase genes we decided to concentrate on just one, which does not encode a laccase-like protein. We have chosen gene [http://www.arabidopsis.org/servlets/TairObject?type=sequence&id=123870/ AT5G48100.1] from A. thaliana Col-0 and moved on with further research. In the next step we used the [http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi/ eFP Browser][2] to see where our chosen laccase is expressed. We found out that it is expressed in the seeds and therefore available in the siliques as it is indicated in the following picture:
With this bioinformatic research we set the basis for the following laboratory work on a laccase from a higher eukaryote.
Bacillus halodurans
Bacillus halodurans is a gram-positive rod-shaped bacterium which can be found in the soil. It is motile and able to develope spores. Containing unique genes, sigma factors and OLE RNAs, it can adapt to alkaline or alcoholic environments. It is able to synthesize a two-peptide antibiotic called haloduracin which can already be produced biotechnologically.[3] We found a published [http://www.ncbi.nlm.nih.gov/pubmed/15293032 article] in which a multicopper oxidase with potential laccase activity and a molecular weight of 56 kDa is described. The gene product was able to oxidize 2,2’-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS), 2,6-dimethoxyphenol (2,6-DMP) and syringaldazine (SGZ).[4] This putative laccase is interesting for us, because its pH optimum is between 7.5 and 8. So this is close to the prevailing pH of the wastewater in a wastewater treatment plant (pH 7). The strain [http://www.dsmz.de/catalogues/details/culture/DSM-27.html B. halodurans C-125] we use is classified as a GRAS organism on [http://www.bvl.bund.de/SharedDocs/Downloads/06_Gentechnik/register_datenbanken/organismenliste.html?nn=1484542 basic biosafety level 1]. For further information on our lbh1 characterization click here.
Bacillus pumilus
[http://www.dsmz.de/catalogues/details/culture/DSM-27.html Bacillus pumilus DSM 27 (ATCC 7061)] is one of our bacterial donators for laccase genes. This organism is classified as a GRAS organism on [http://www.bvl.bund.de/SharedDocs/Downloads/06_Gentechnik/register_datenbanken/organismenliste.html?nn=1484542 basic biosafety level 1]. Bacillus pumilus is also a gram-positive soil bacterium. It is already used for alkaline protease production, in environmental decontamination of dioxins and in the baking industry. The protein [http://www.ncbi.nlm.nih.gov/protein/ZP_03054403 CotA] which we use is described as spore coat protein and has a molecular weight of 58.6 kDa. We chose this laccase because of its catalytic activity shown for 2,2’-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid (ABTS), 2,6-dimethoxyphenol (2,6-DMP), syringaldazine (SGZ) and many other substrates ([http://www.biomedcentral.com/1472-6750/11/9 look here for the publication]).[5] So the enzyme seemed an appropriate laccase for examining the activity on our substrates. For further information about our characterization of [http://www.ncbi.nlm.nih.gov/protein/ZP_03054403 CotA] click here.
Escherichia coli
Another laccase which catched our attention is the enzyme [http://www.ncbi.nlm.nih.gov/protein/85674340 CueO] from Escherichia coli. The bacterium naturally occurs in human and animal intestine. In laboratory it is a model organism and the most widely used organism in molecular genetics. The about 1-6 µm long bacterium has a laccase with a high potential for our approach.
It is described that the laccase [http://www.ncbi.nlm.nih.gov/protein/85674340 CueO] from E. coli W3110 is able to oxidize different PAHs (polycyclic aromatic hydrocarbons). An oxidation activity for the crude laccase was measured for fluorene, acenaphthylene, phenanthrene and benzo[a]anthracen [6]). The laccase has a predicted molecular weight of 53 kDa. Our aim is to produce and characterize this laccase with regard to the plan to develop a degradation system using immobilized laccases. After blasting this laccase we found out that E. coli BL21(DE3) has this laccase, too. Therefore we isolated the laccase CueO from E. coli BL21(DE3) because this strain is available in our lab. For consistently names we call this laccase ecol in our wiki. For further information about our characterization of CueO look here.
Thermus thermophilus
Thermus thermophilus is a gram-negative eubacterium. It is classified as an organism of the [http://www.bvl.bund.de/SharedDocs/Downloads/06_Gentechnik/register_datenbanken/organismenliste.html?nn=1484542 basic biosafety level 1]. The bacterium is as the name suggests extremely thermophilic and has an optimal growth temperature at about 65 °C. Due to its cell characteristics this organism can grow on temperatures up to 85 °C.[7] This properties are caused by very thermostable proteins which lead to a high interest in many proteins from different Thermus strains for laboratory research. The most famous strain is probably Thermophilus aquaticus which was isolated from water of hot springs. The strain has an interesting polymerase, the widely used Taq polymerase, a very thermostable protein. In our project we are interested in another protein of Thermus thermophilus, called laccase. The first description of the [http://www.ncbi.nlm.nih.gov/pubmed/15999224 Tth-laccase] from T. thermophilus was in year 2005. This multi copper blue oxidase (53 kDa), short laccase, shows an oxidative activity on ABTS and SGZ.[8] Like the most proteins of T. thermophilus this enzyme presents a high stability under harsh industrial conditions. Due to its skills and properties our attention was drawn to this laccase. We decided to isolate the sequence from the strain [http://www.dsmz.de/catalogues/details/culture/DSM-7039 T. thermophilus HB27] and to develope a degradation system against micro-contaminants using this laccase immobilized. More information about the characterization of our produced Tth-laccase (TTHL) you find here.
Trametes versicolor
Trametes versicolor is a common mushroom which is very widespread all over the world, so it is also classified as an organism of the [http://www.bvl.bund.de/SharedDocs/Downloads/06_Gentechnik/register_datenbanken/organismenliste.html?nn=1484542 basic biosafety level 1]. It is described that Trametes versicolor has four genes for different laccase isoenzymes. The substrate specifies were described as mostly similar but with certain variations in the oxidation rate of polymeric substrates. Laccases from Trametes versicolor exhibits a high redox potential and are shown to remove substrates such as phenol and bisphenol A. Furthermore they are able to degrade endocrine disrupters (natural estrogens, 17β-estradiol (E2), estriol (E3),estrone (E1) and the synthetic estrogen 17α-ethinylestradiol (EE2)).[9] For further information about the production of laccases from Trametes versicolor click here and for information about the characterization of [http://www.sigmaaldrich.com/catalog/product/sigma/51639?lang=de®ion=DE TVEL0] which we used as reference and positive control click here.
References
[1] The Arabidopsis Information Resource (TAIR), www.arabidopsis.org/aboutarabidopsis.html, on www.arabidopsis.org, Oct 24, 2000
[2] Winter et al. (2007). Arabidopsis eFP Browser at bar.utoronto.ca. PLosOne 2(8): e718.
[3] Ruijssenaars HJ, Hartmans S (2004). A cloned Bacillus halodurans multicopper oxidase exhibiting alkaline laccase activity. Applied Genetics and Molecular Biotechnology 65:177-182
[4] Danesh A, Mamo G, Mattiasson B (2011). Production of haloduracin by Bacillus halodurans using solid-state fermentation. Biotechnology Letters 33(7):1339-44.
[5] Reiss, R., J. Ihssen, et al. (2011). "Bacillus pumilus laccase: a heat stable enzyme with a wide substrate spectrum." BMC Biotechnol 11: 9.
[6] Zeng, J., X. Lin, et al. (2011). Oxidation of polycyclic aromatic hydrocarbons by the bacterial laccase CueO from E. coli. Appl Microbiol Biotechnol 89(6): 1841-1849.
[7] Wallace JG, Zhou Z, Breaker PR (2012). OLE RNA protects extremophilic bacteria from alcohol toxicity. Nucleic Acids Research 40(14):6898-6907.
[8] Miyazaki, K. (2005). A hyperthermophilic laccase from Thermus thermophilus HB27. Extremophiles 9(6): 415-425.
[9] Bourbonnais, R., M. G. Paice, et al. (1995). Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2,2'-azinobis(3-ethylbenzthiazoline-6-sulfonate) in kraft lignin depolymerization. Appl Environ Microbiol 61(5): 1876-1880.
| 55px | | | | | | | | | | |
 "
"