Team:HokkaidoU Japan/Notebook/plastic Week 8
From 2012.igem.org
(→Plasmid extraction) |
|||
| (48 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
<div id="hokkaidou-column-main"> | <div id="hokkaidou-column-main"> | ||
<!-- DO NOT EDIT OVER THIS LINE @iTakeshi --> | <!-- DO NOT EDIT OVER THIS LINE @iTakeshi --> | ||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==August | + | ===August 20th=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | ==Digestion== | + | ====Digestion==== |
| - | < | + | We digested 3 samples.<br> |
| - | + | Digested of PhaC and pSB1C3 by XbaI and SpeI. | |
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
| Line 32: | Line 31: | ||
|} | |} | ||
| - | + | ||
| + | Digested of PhaA by XbaI site and SpeI site. | ||
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
|- | |- | ||
| Line 55: | Line 55: | ||
| - | + | And digested PhaB by XbaI site and SpeI site. | |
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
|- | |- | ||
| Line 76: | Line 76: | ||
|20 ul | |20 ul | ||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
</div></div> | </div></div> | ||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | + | ===August 21st=== | |
| - | ==August | + | <div class="hokkaidou-section"> |
| - | <div | + | |
| - | = | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
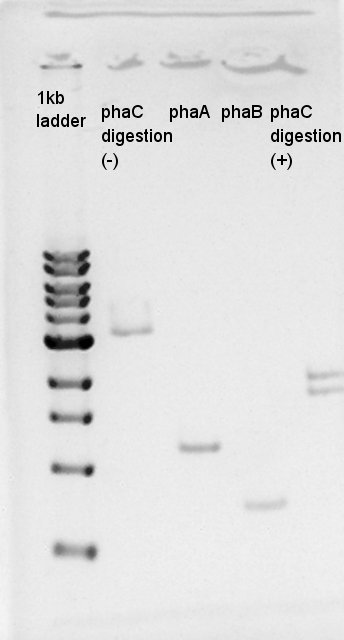
[[image:HokkaidoU2012 120821PhaA PhaB PhaC digestion okamura.jpg|thumb|digestion result]] | [[image:HokkaidoU2012 120821PhaA PhaB PhaC digestion okamura.jpg|thumb|digestion result]] | ||
| + | ====Electrophoresis==== | ||
| + | We confirmed whether PhaC was digested correctly, and phaA and phaB were done PCR correctly by electrophoresis. | ||
| - | + | ====Gel extraction==== | |
| - | + | We confirmed succession of digestion by electrophoresis, then DNA were extracted from TBE gel. | |
| - | + | And we used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution. | |
| - | + | <br style="line-height: 0; clear: both;" /> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | ==Gel extraction== | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | </ | + | |
| - | + | ||
</div></div> | </div></div> | ||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | + | ===August 22nd=== | |
| - | ==August | + | <div class="hokkaidou-section"> |
| - | <div | + | |
| - | = | + | |
| - | + | ||
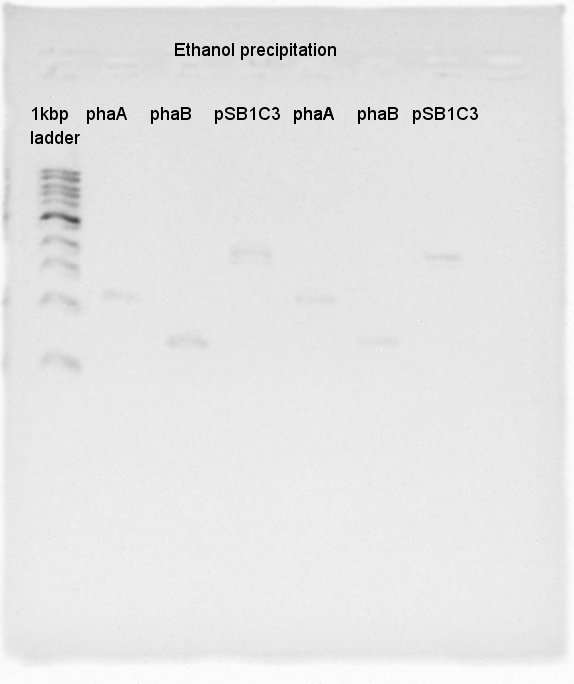
[[image:HokkaidoU 2012 120822PhaA PhaB pSB1C3 ETOH.jpg|thumb|Ethanol precipitation result]] | [[image:HokkaidoU 2012 120822PhaA PhaB pSB1C3 ETOH.jpg|thumb|Ethanol precipitation result]] | ||
| + | ====Ethanol precipitation==== | ||
| + | The DNA extracted at August 21st were condensed by Ethanol precipitation. | ||
| + | ====Ligation==== | ||
| + | We ligated phaA with pSB1C3 and phaB with pSB1C3. | ||
| - | + | ====Transformation==== | |
| - | + | The ligated DNA were transformed into E. coli (strain:DH5α). | |
| - | == | + | E. coli solution was spread on LBC. |
| - | + | <br style="line-height: 0; clear: both;" /> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | </ | + | |
| - | + | ||
| - | + | ||
| - | + | ||
</div></div> | </div></div> | ||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==August | + | ===August 23rd=== |
| - | <div | + | <div class="hokkaidou-section"> |
| - | = | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
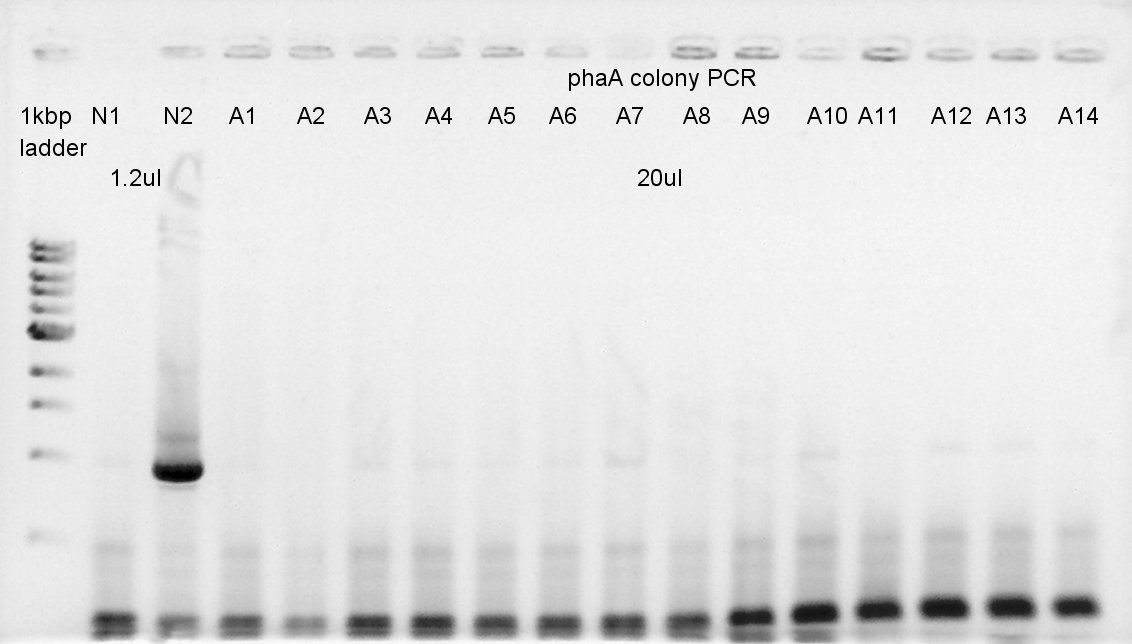
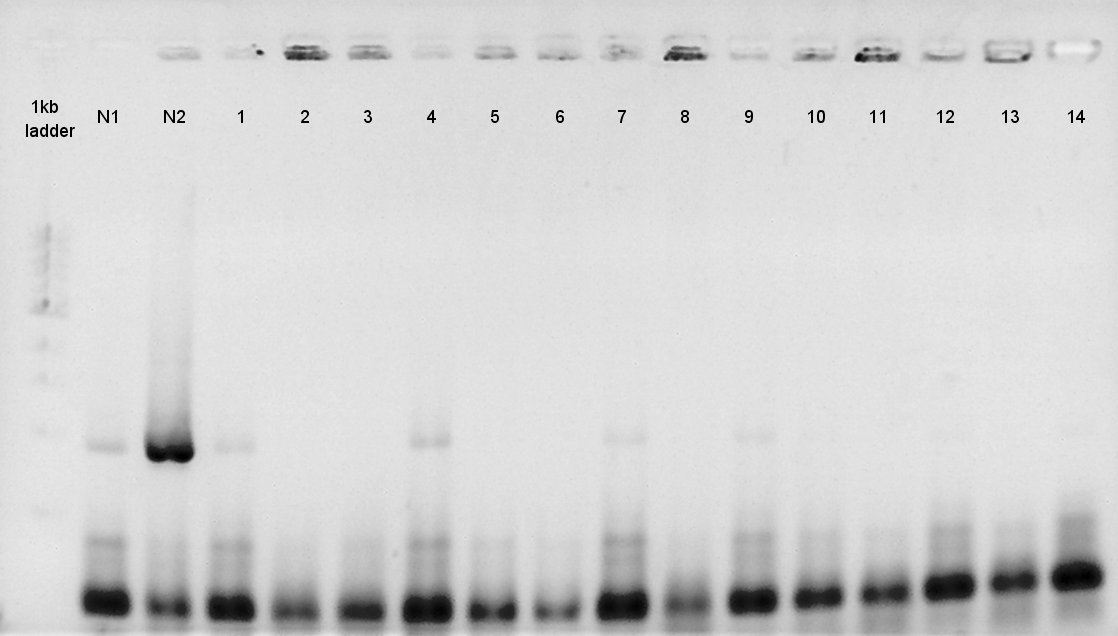
[[image:HokkaidoU 120823 PhaAcolop1 14.jpg|thumb|Colony PCR result]] | [[image:HokkaidoU 120823 PhaAcolop1 14.jpg|thumb|Colony PCR result]] | ||
| - | |||
[[image:HokkaidoU 120823PhaBcolop1 14.jpg|thumb|Colony PCR result]] | [[image:HokkaidoU 120823PhaBcolop1 14.jpg|thumb|Colony PCR result]] | ||
| - | </ | + | ====Colony PCR==== |
| - | + | We confirmed whether the ligation at August 22nd went well by colony PCR result. | |
| + | <br style="line-height: 0; clear: both;" /> | ||
</div></div> | </div></div> | ||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==August | + | ===August 24th=== |
| - | <div> | + | <div class="hokkaidou-section"> |
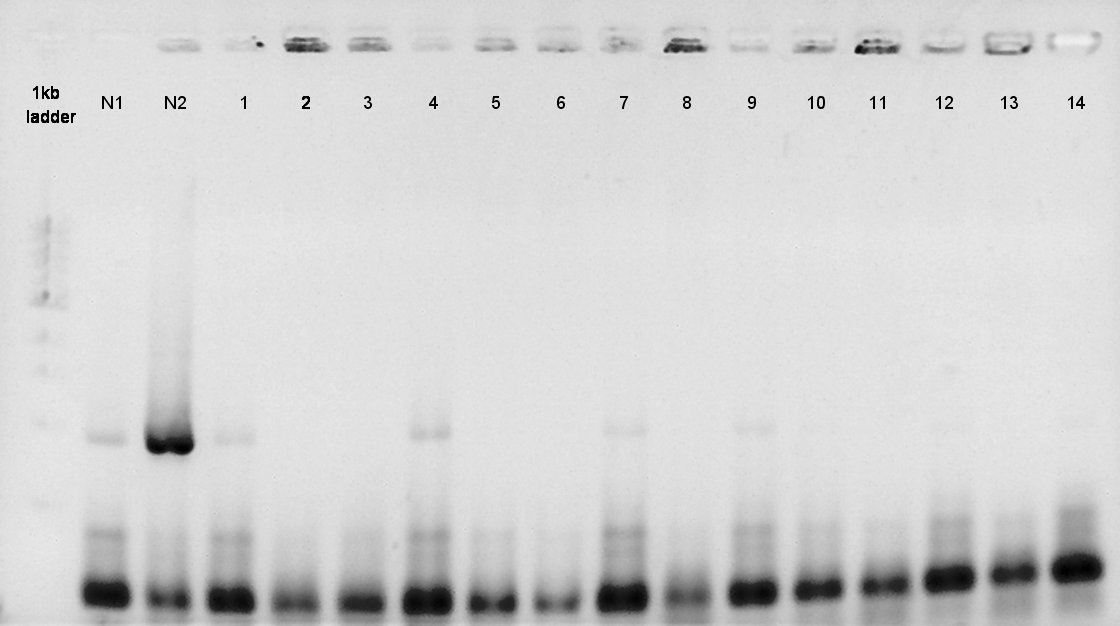
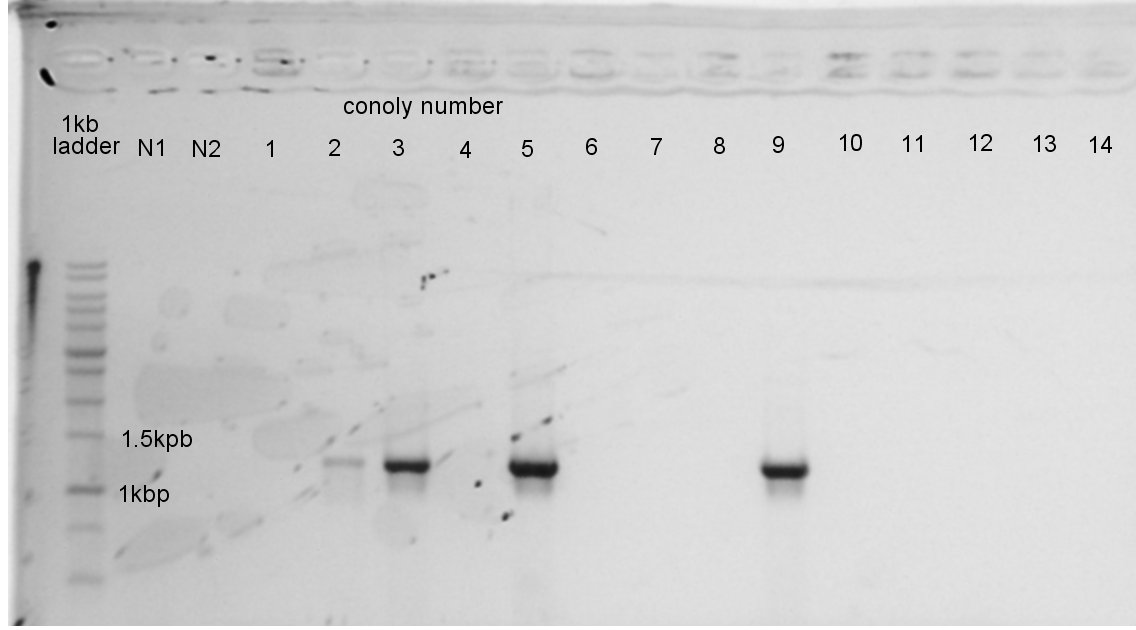
| - | + | [[image:HokkaidoU2012 120824 PhaA coloP.jpg|thumb|Colony PCR result]] | |
| - | + | [[image:HokkaidoU2012 120824 PhaB coloP.jpg|thumb|Colony PCR result]] | |
| + | ====Colony PCR==== | ||
We did colony PCR of PhaA and PhaB again. | We did colony PCR of PhaA and PhaB again. | ||
| - | + | From the result, we thought that we failed to ligate PhaA with pSB1C3 and PhaB with pSB1C3. So we decided to ligated them again. | |
| - | + | ||
| - | + | ||
| - | + | ||
| + | ====Ligation==== | ||
| + | We ligated phaA and phaB with pSB1C3. | ||
| + | <br style="line-height: 0; clear: both;" /> | ||
</div></div> | </div></div> | ||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==August | + | ===August 25th=== |
| - | <div> | + | <div class="hokkaidou-section"> |
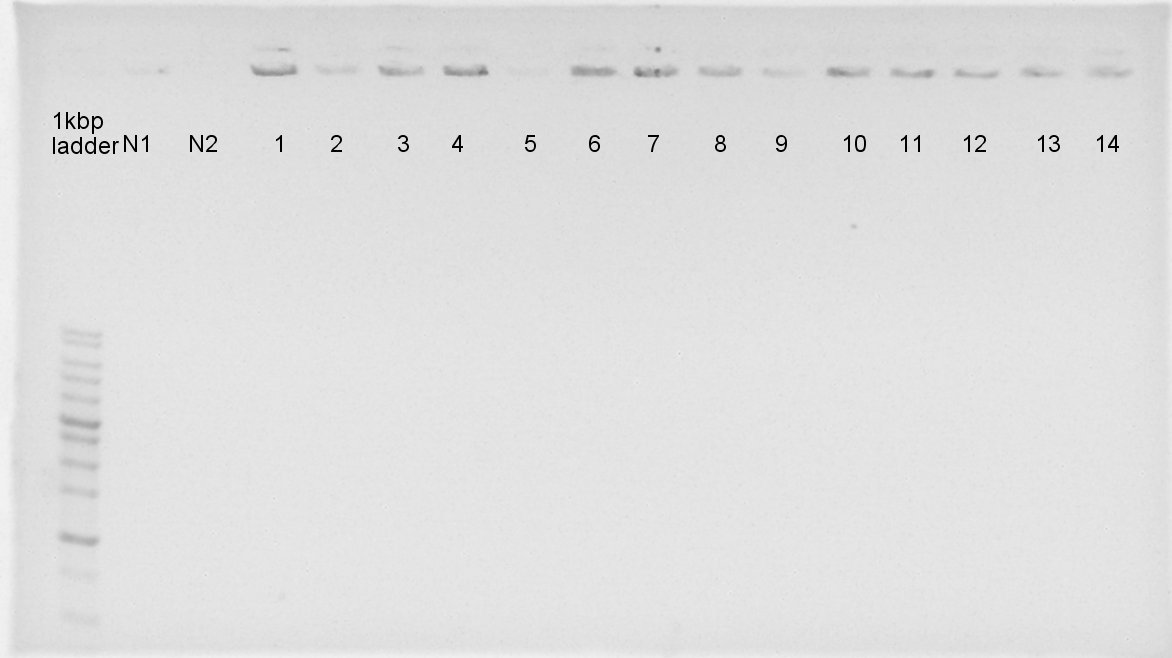
| - | + | [[image:HokkaidoU120825 phaAcolonyPCR.jpg|thumb|Colony PCR result]] | |
| - | + | [[image:HokkaidoU2012 120825 phaBcolonyPCR.jpg|thumb|Colony PCR result]] | |
| - | + | ||
| - | + | ||
| - | == | + | ====Colony PCR==== |
| - | + | We confirmed the length of PhaA on pSB1C3 and PhaB on pSB1C3 by colony PCR.<br> | |
| - | + | The result showed only PhaA and pSB1C3 were ligated correctly. | |
| - | < | + | |
| + | ====Liquid culture==== | ||
| + | We started to incubate bacteria holds RBS (B0034). | ||
| + | ====Digestion==== | ||
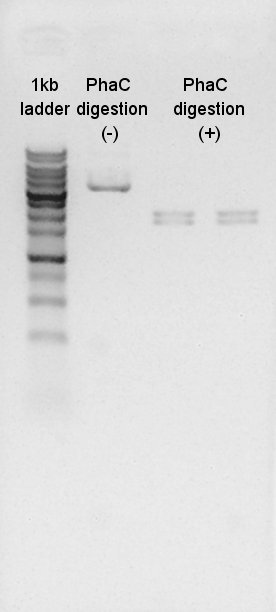
| + | We digested PhaC(BBa_K342001) by XbaI and SpeI. The result shows that the digestion was succeeded. | ||
| + | |||
| + | [[image:HokkaidoU2012 120825 phaCdigestion.jpg|thumb|digestion result]] | ||
| + | <br style="line-height: 0; clear: both;" /> | ||
</div></div> | </div></div> | ||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==August 26th== | + | ===August 26th=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | ==Plasmid extraction== | + | [[image:HokkaidoU2012 120826 RBS&PhaA.jpg|thumb|plasmid extraction result]] |
| - | + | ====Plasmid extraction==== | |
| - | The plasmid of RBS (BBa_B0034) were extracted.<br/>And then we got | + | The plasmid of RBS (BBa_B0034) were extracted.<br/>And then we got 50 ul DNA solution. |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | ====Digestion==== | ||
| + | RBS (BBa_B0034) was digested with SpeI and PstI restriction sites.<br/>And also PhaC (BBa_K342001) was digested by XbaI and PstI. | ||
| + | ====Liquid culture==== | ||
| + | We started to incubate. | ||
| + | <br style="line-height: 0; clear: both;" /> | ||
| + | </div></div> | ||
<!-- DO NOT EDIT UNDER THIS LINE @iTakeshi --> | <!-- DO NOT EDIT UNDER THIS LINE @iTakeshi --> | ||
Latest revision as of 04:00, 27 September 2012
Contents |
August 20th
Digestion
We digested 3 samples.
Digested of PhaC and pSB1C3 by XbaI and SpeI.
| DNA solution PhaC | 12 ul |
| XbaI | 1 ul |
| SpeI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 4 ul |
| Total | 20 ul |
Digested of PhaA by XbaI site and SpeI site.
| DNA solution PhaA | 7 ul |
| XbaI | 1 ul |
| SpeI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 9 ul |
| Total | 20 ul |
And digested PhaB by XbaI site and SpeI site.
| DNA solution PhaB | 7 ul |
| XbaI | 1 ul |
| SpeI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 9 ul |
| Total | 20 ul |
August 21st
Electrophoresis
We confirmed whether PhaC was digested correctly, and phaA and phaB were done PCR correctly by electrophoresis.
Gel extraction
We confirmed succession of digestion by electrophoresis, then DNA were extracted from TBE gel.
And we used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution.
August 22nd
August 23rd
August 24th
August 25th
Colony PCR
We confirmed the length of PhaA on pSB1C3 and PhaB on pSB1C3 by colony PCR.
The result showed only PhaA and pSB1C3 were ligated correctly.
Liquid culture
We started to incubate bacteria holds RBS (B0034).
Digestion
We digested PhaC(BBa_K342001) by XbaI and SpeI. The result shows that the digestion was succeeded.
August 26th
Plasmid extraction
The plasmid of RBS (BBa_B0034) were extracted.
And then we got 50 ul DNA solution.
Digestion
RBS (BBa_B0034) was digested with SpeI and PstI restriction sites.
And also PhaC (BBa_K342001) was digested by XbaI and PstI.
Liquid culture
We started to incubate.
 "
"