Team:Bielefeld-Germany/Results/trametis
From 2012.igem.org
(→Immobilisation) |
(→Immobilisation) |
||
| Line 82: | Line 82: | ||
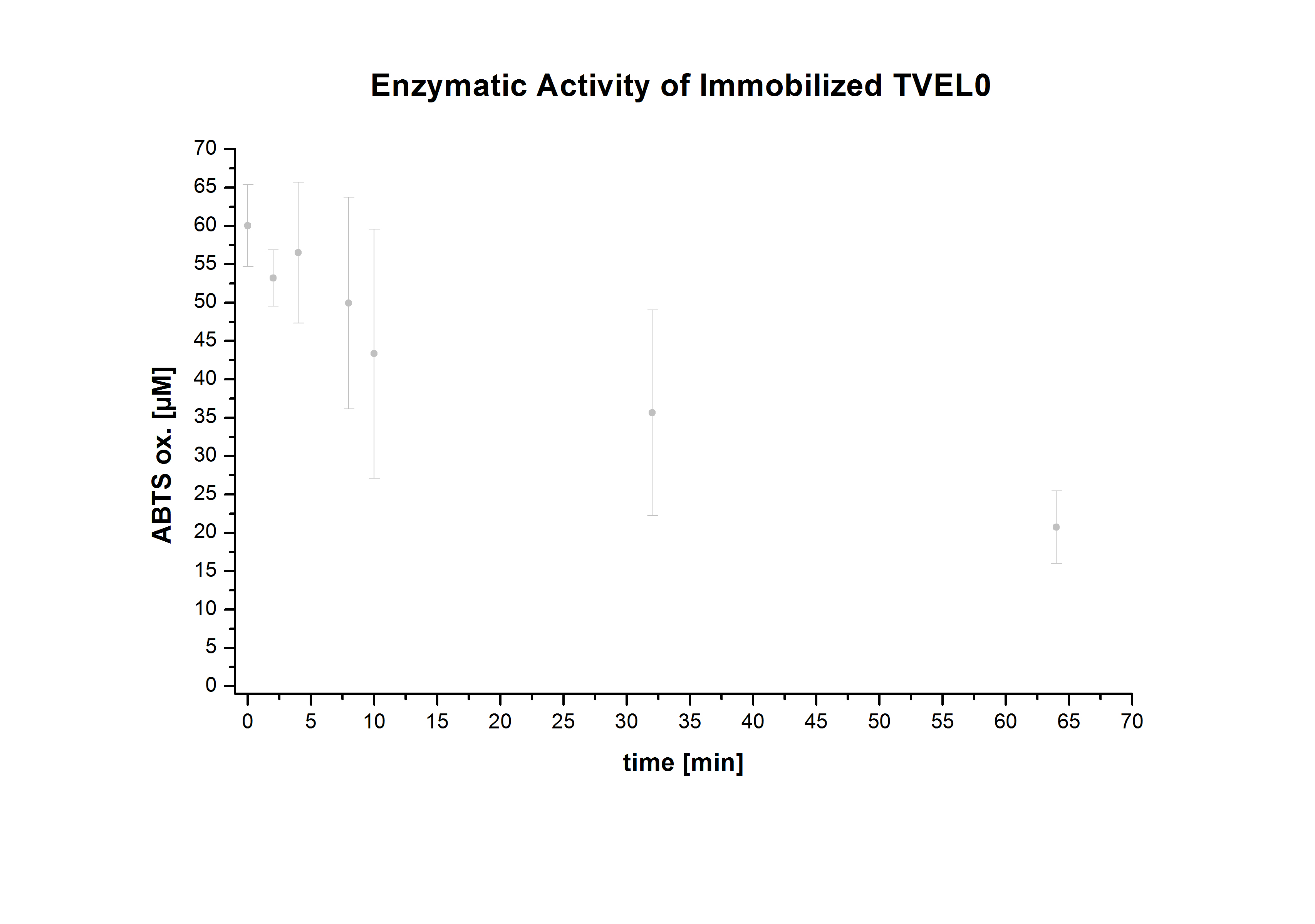
| - | Figure 7 shows the enzymatic activity of immobilized TVEL, measured using 0.1 mM ABTS at 25°C over a time period of 65min. It can be seen that the activity rises directly, so that the enzymatic activity already reaches a relative high | + | Figure 7 shows the enzymatic activity of immobilized TVEL, measured using 0.1 mM ABTS at 25°C over a time period of 65min. It can be seen that the activity rises directly, so that the enzymatic activity already reaches a relative high level during the sample preparation. |
<br style="clear: both" /> | <br style="clear: both" /> | ||
Revision as of 04:00, 27 September 2012

Summary
TVEL0 was acquired commercially from [http://www.sigmaaldrich.com/catalog/product/sigma/53739?lang=de®ion=DE Sigma-Aldrich] for creating a standard in enzyme activity and further comparisons to the produced recombinant laccases. TVEL0 was characterized in terms of it´s activity under different pH, CuCl2 ABTS, MEOH and acetonitrile concentrations. The protein concentrations of all purified laccases were adjusted to the amount of diluted TVEL0 in each sample, respectively.
Contents |
TVEL0 Activity Tests
Initial Activity Test
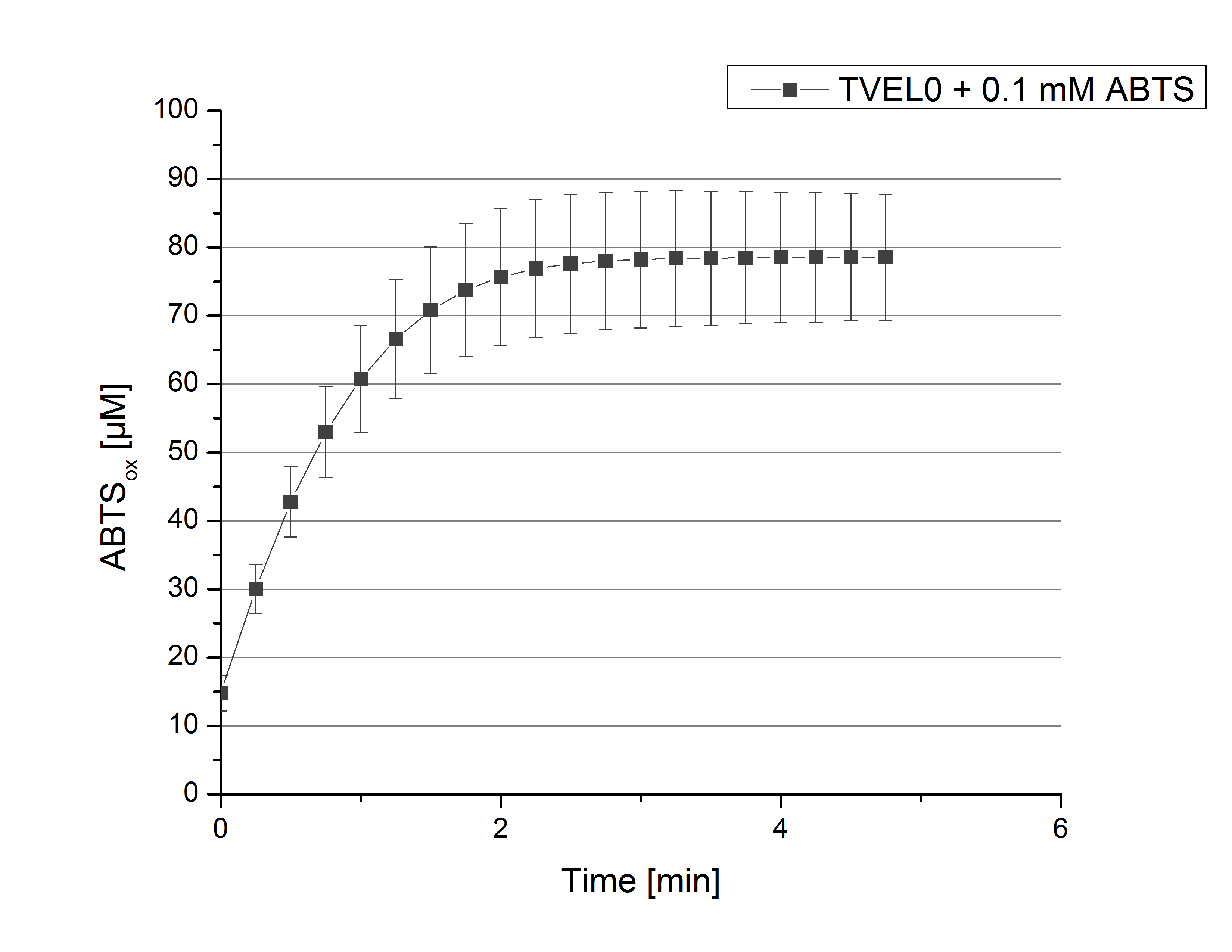
To standardize activity test methods used for this project, a [http://www.sigmaaldrich.com/catalog/product/sigma/51639?lang=de®ion=DE laccase from Trametes versicolor] (TVEL0) was used. The optimal composition for activity measurements contains 140 µL of 0.03 mg mL-1 concentrated TVEL0 laccase solution, 0.1 mM ABTS and 100 mM sodium acetate buffer to a final volume of 200 µL. With this approach ABTS oxidation activity of TVEL0 was measured over a time period of 5 minutes at 25°C. The saturation of the reaction was reached after 3 minutes when ~80% ABTS got oxidized (see Figure 1). This result proved the activity measurement method and can therefore be used as a positive control.
Optimal pH of TVEL0
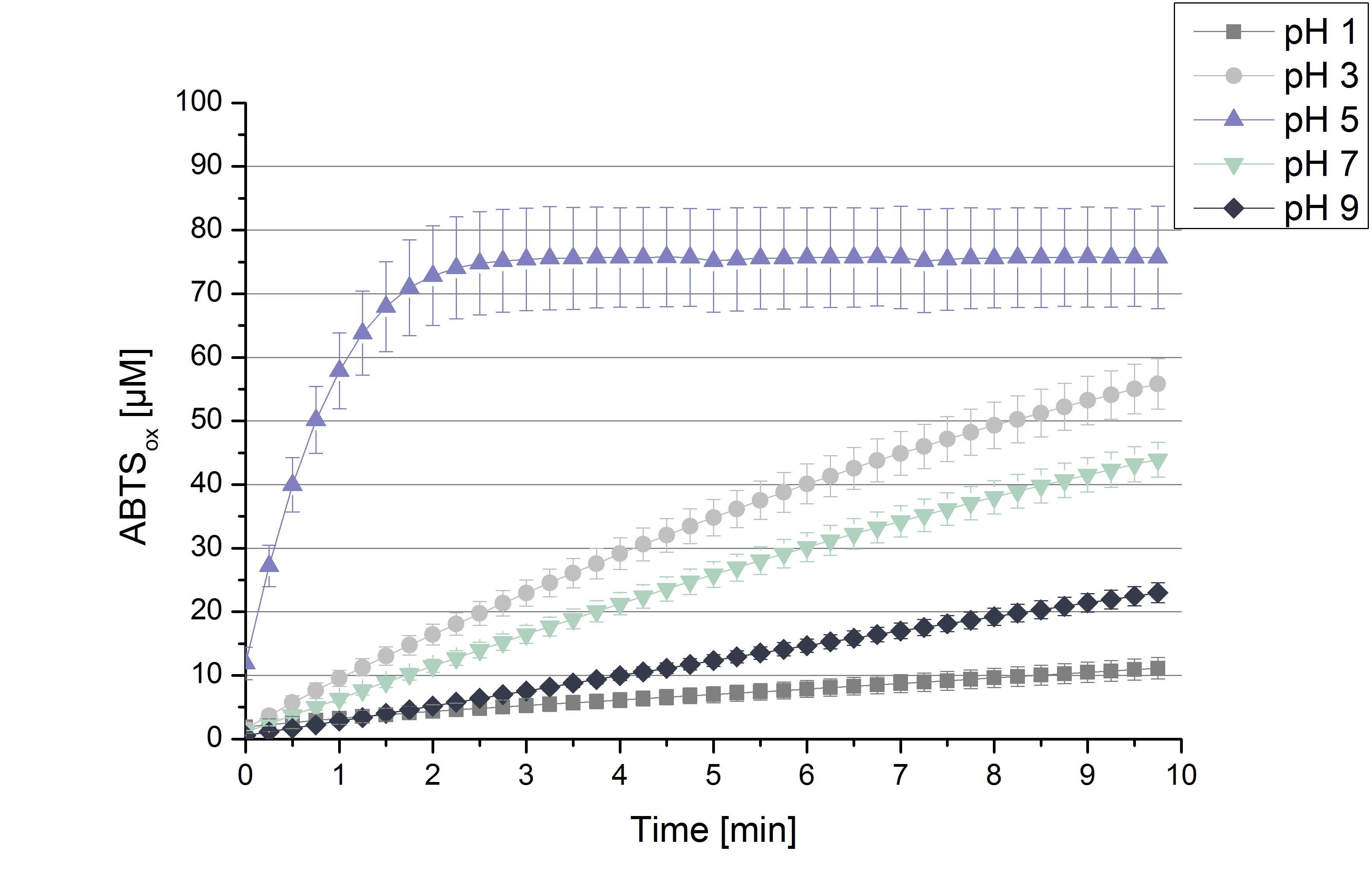
To determine the activity of TVEL0 in regard of optimal pH conditions different sodium acetate buffer pHs were under consideration for activity tests. Ranging from pH 1 to pH 9 the standardized activity setup of 140 µL of 0.03 mg mL-1 concentrated TVEL0 laccase solution, 0.1 mM ABTS and 100 mM sodium acetate to a final volume of 200 µL was used. Only at pH 5 a saturation in ABTSox can be reached (see Figure 2). As in the initial activity test above the maximal amount of ABTSox accounts ~80%. In summary the optimal pH for TVEL0 activity to oxidize ABTS is pH 5.
TVEL0 activity depending on different ABTS concentrations
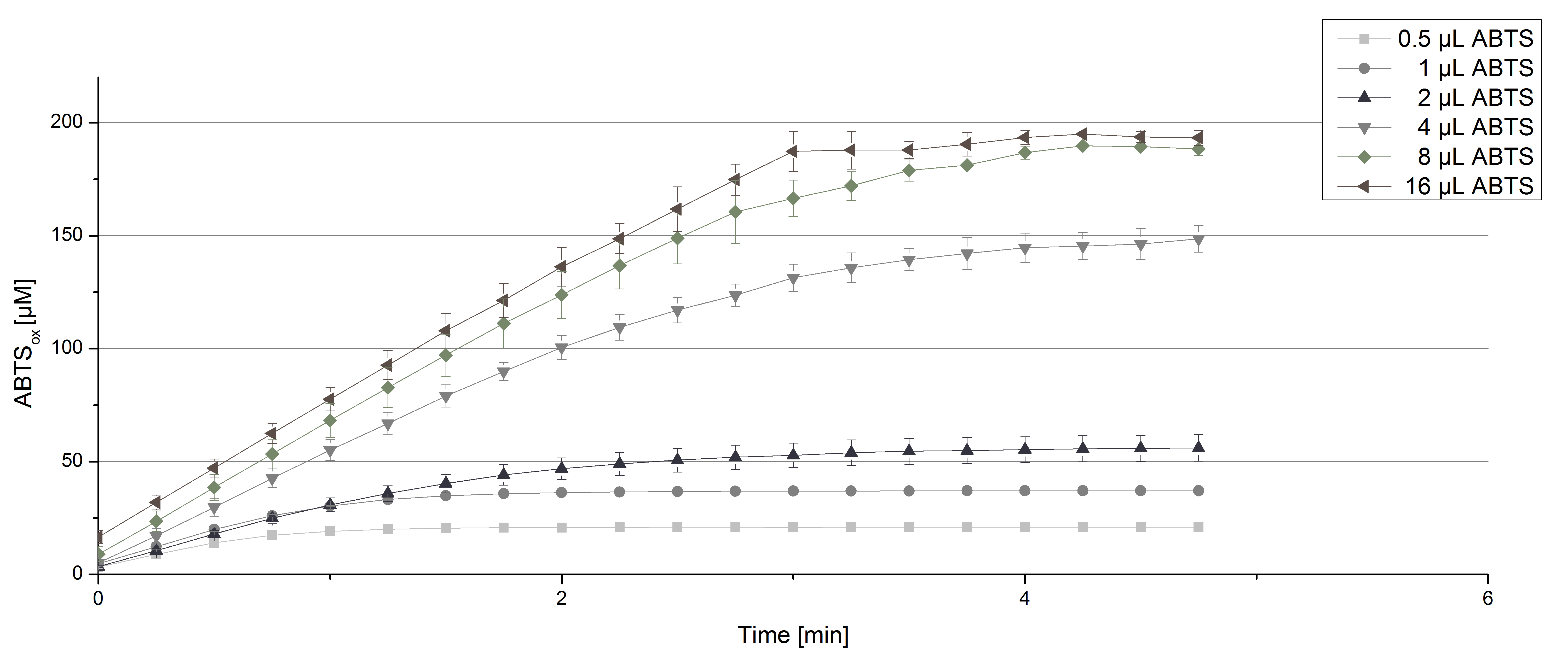
TVEL0 laccase were tested using different amounts of ABTS to calculate KM and Kcat values. Instead of using 140 µL of a 0.03 mg mL-1 TVEL0 protein solution, the amount was quartered to 35 µL of this solution. Reducing the enzyme concentration was necessary to detect the change in OD420 at the beginning of the reaction. The standardized measurement setup as described above was used only with different amounts of ABTS. As expected, the amount of oxidized ABTS increased in dependence of the amount of ABTS used (Figure 3).
Impact of MeOH and acteonitrile on TVEL0
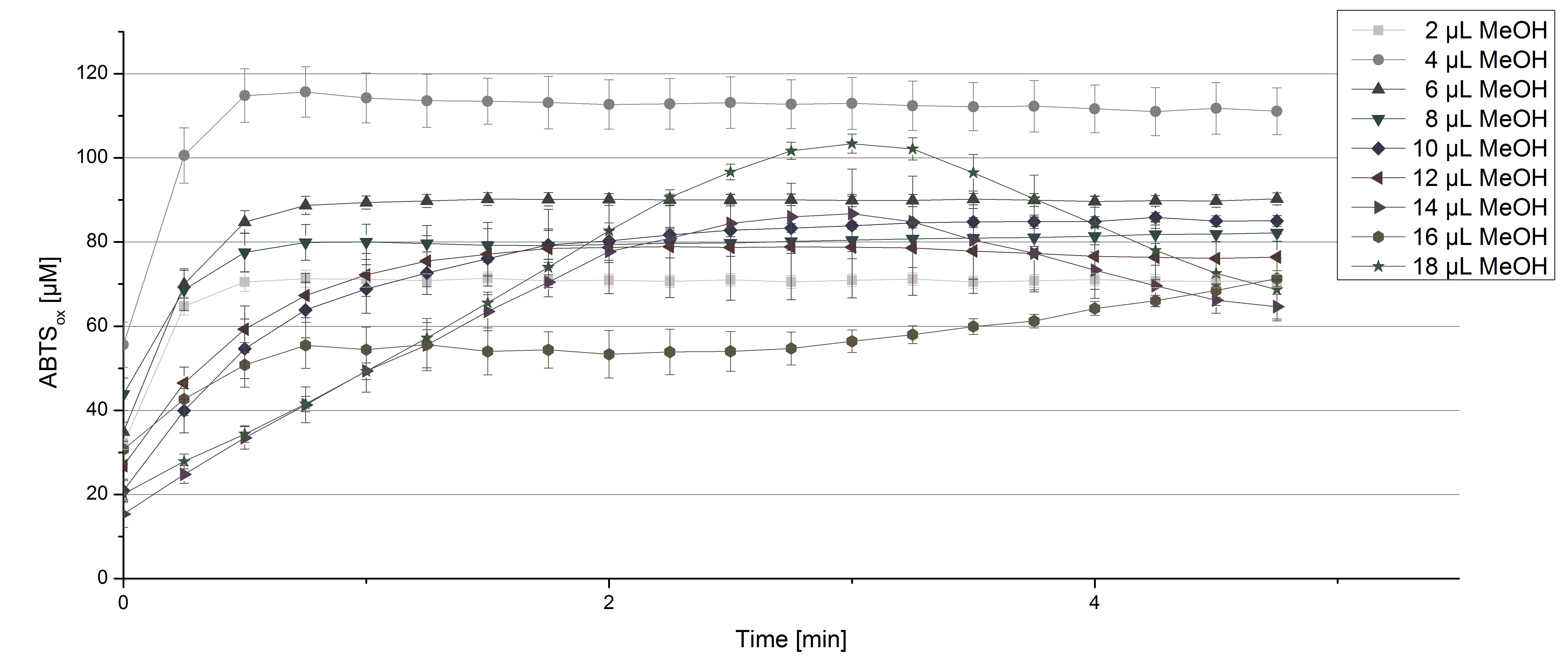
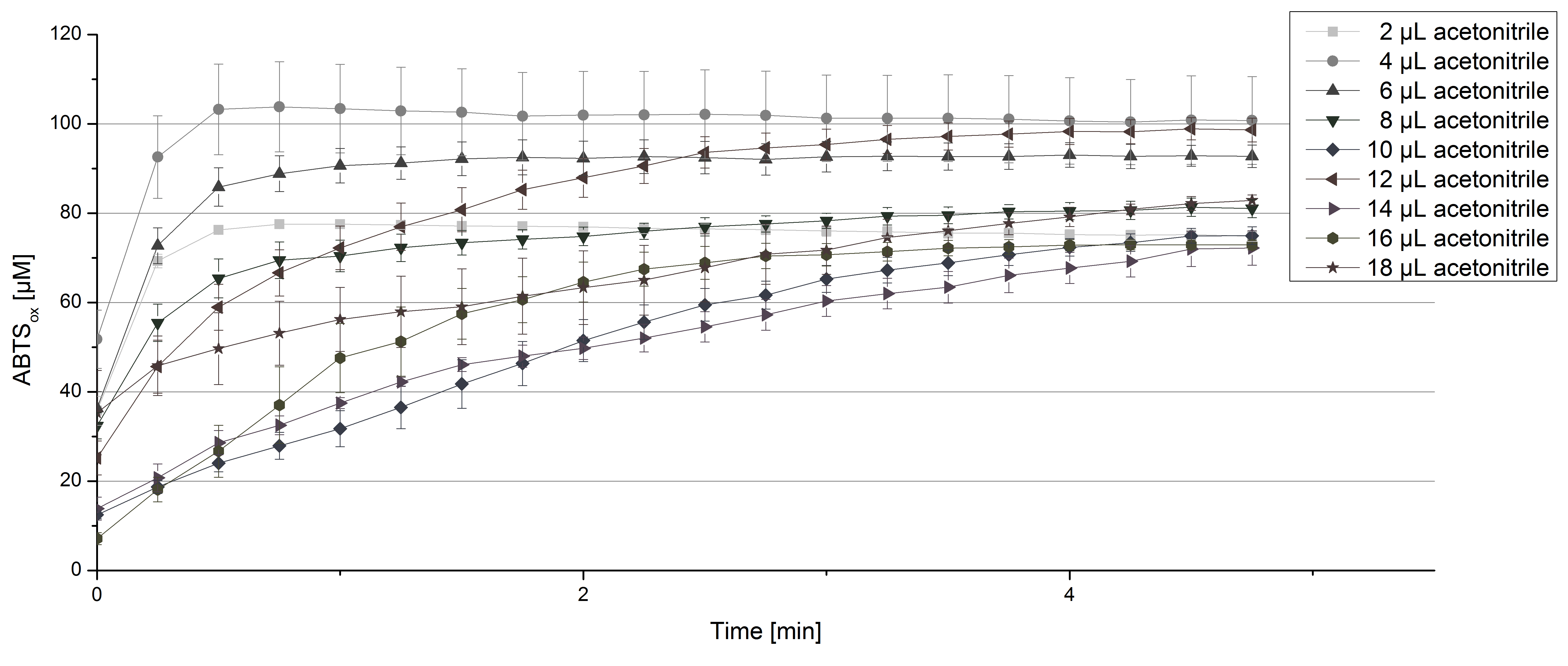
For substrate analysis the usage of MeOH and acetonitrile is necessary to dissolve the substrates. To make sure TVEL0 laccase activity is not affected by these solvents activity tests using different amounts of MeOH and acetonitrile were done. An increase in MeOH or acetonitrile amount affects the activity of TVEL0, but leads to a saturation curve in most cases. Regarding tests with MeOH an addition of 14 µL of MeOH or more causes a loss of saturation (see Figure 4). Usage of 12 µL acetonitrile or more results in the saturation curves getting disordered (see Figure 5). Still, activity is detectable in all cases, thus usage of MeOH and acetonitrile for substrate analysis is possible.
Immobilisation
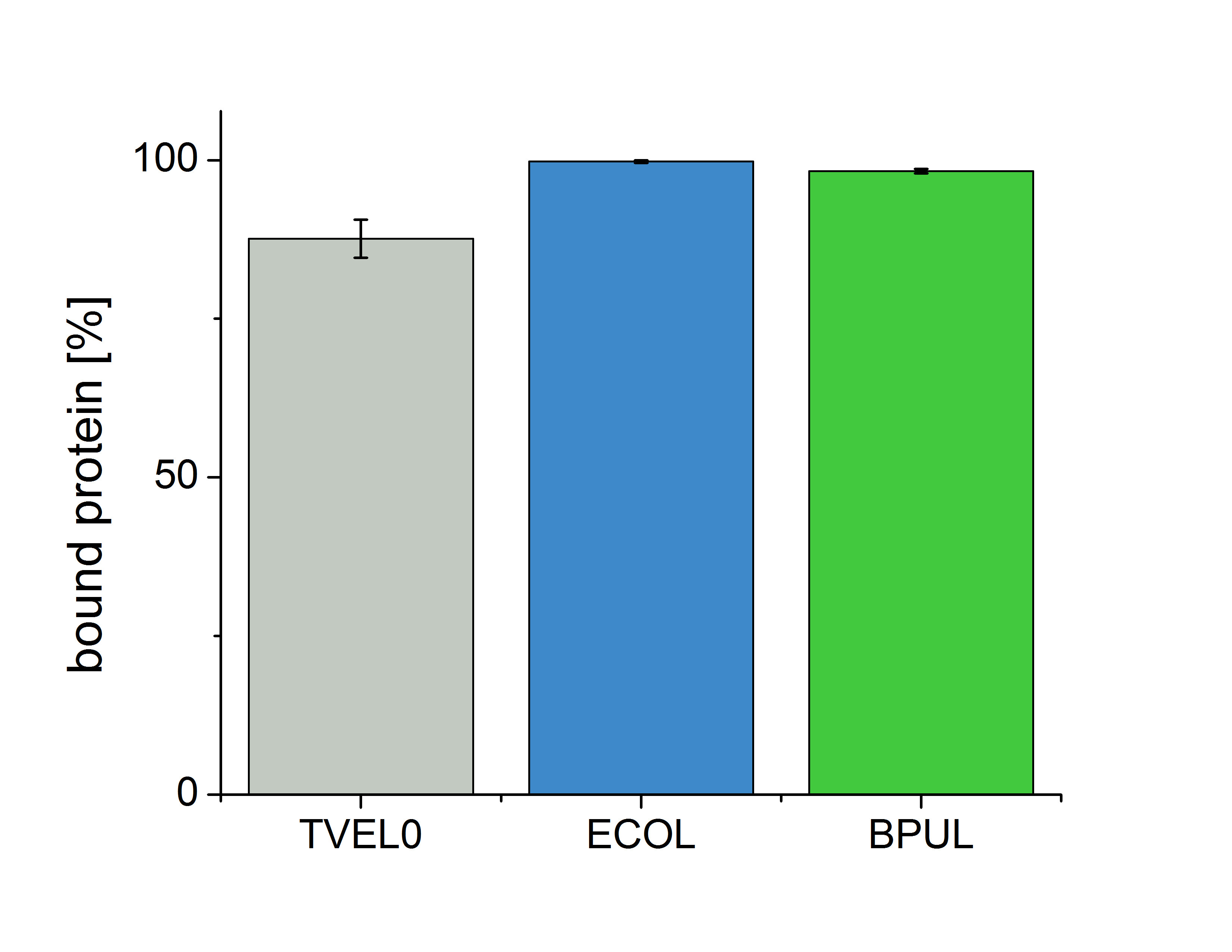
Figure 6 shows the percentage of laccases in the supernatant after incubation with CPC-beads, relative to the original concentration . The concentration of laccases in the supernatant after incubation was measured using Roti®-Nanoquant. The results show that 12.4% of TVEL0 remained in the supernatant. This indicates a relatively high binding capacity of BPUL on CPC-beads.
Figure 7 shows the enzymatic activity of immobilized TVEL, measured using 0.1 mM ABTS at 25°C over a time period of 65min. It can be seen that the activity rises directly, so that the enzymatic activity already reaches a relative high level during the sample preparation.
| 55px | | | | | | | | | | |
 "
"