Team:UNAM Genomics Mexico/Modeling/Heavy Metal AND
From 2012.igem.org

AND's Results
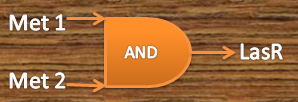
Metal AND
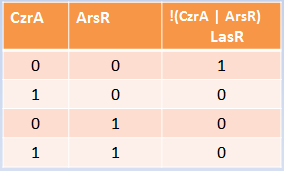
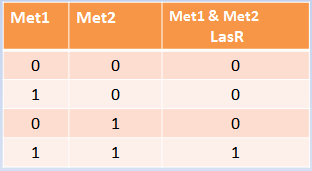
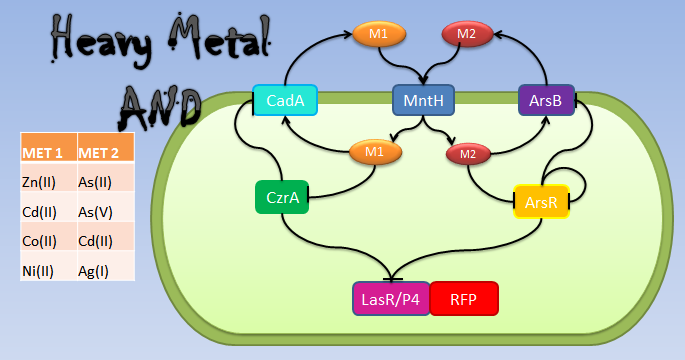
Our system consists of a hybrid promoter which contains binding sites for two transcription factors that act as repressors, CzrA and ArsR. In the presence of Met1, CzrA losses affinity for its binding site.
Similarly, if there is Met2 in the cell, ArsR also dissociates itself from its binding site. Taking into account these facts, unless Met1 and Met2 are present in the culture, we can say that the genes under this hybrid promoter will be repressed, just like a AND gate should function!
Repressors
Inputs
Kappa
There is no metal catabolism, so metals just reach an equilibrium and non-toxic concentration within the cell through exporting it out of the cytosol, maintaining the concentration constant. This makes the behavior of a single burst or a continuous dose similar, because the metal levels in just one dose will be constant since metals won’t be degraded.
Transfer Function
We were interested in modelling the output of our logic gates based on the input. This is called the "Transfer function". Imagine this process like a black box that will give you the dynamic concentration through time of whatever is downstream of the "AND" when you feed it with input data. It doesn't matter if it’s a single input burst or a continuous input.
Our logic AND gate is fully dependent on the intracellular concentrations of the inducers (meaning metal ions).
Then, to accomplish our duty in model, we use the "Transfer Function", for which it is necessary to take into account the regulation and dynamics of the endogenous B. subtilis intake-efflux system that controls the intracellular concentrations of Heavy Metals and Sugars. All of this is going to be the "Black Box".
So, our first task was to reconstruct the regulatory network of B. subtilis for the intake-efflux systems. The regulation data was retrieved from several papers and databases like [http://bsubcyc.org/ http://bsubcyc.org/]
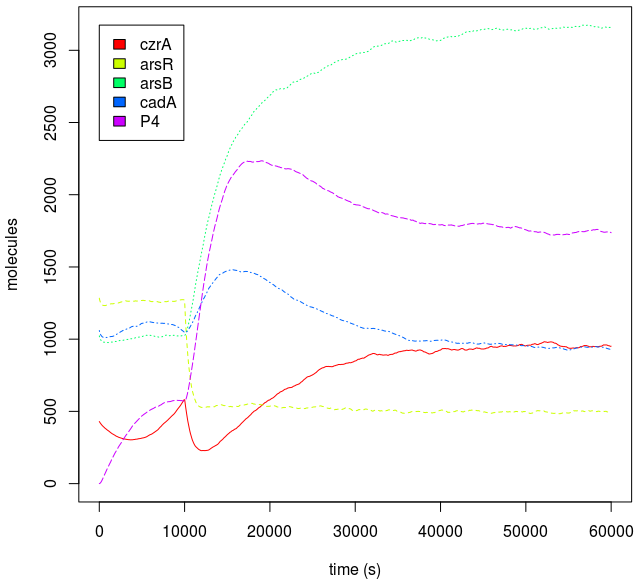
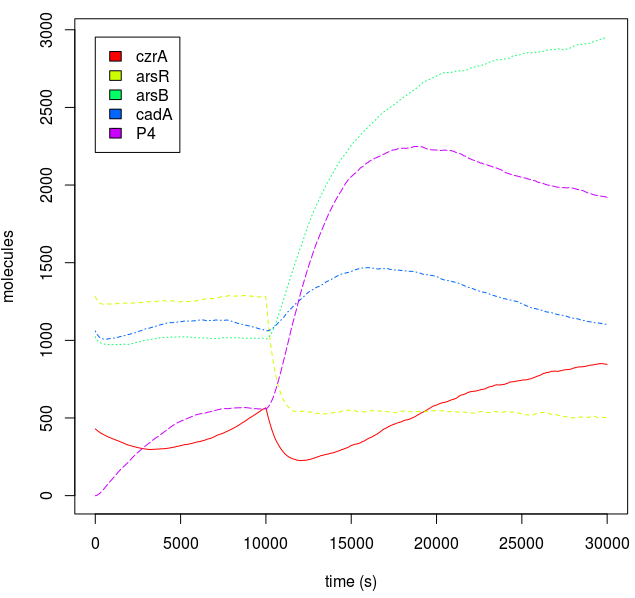
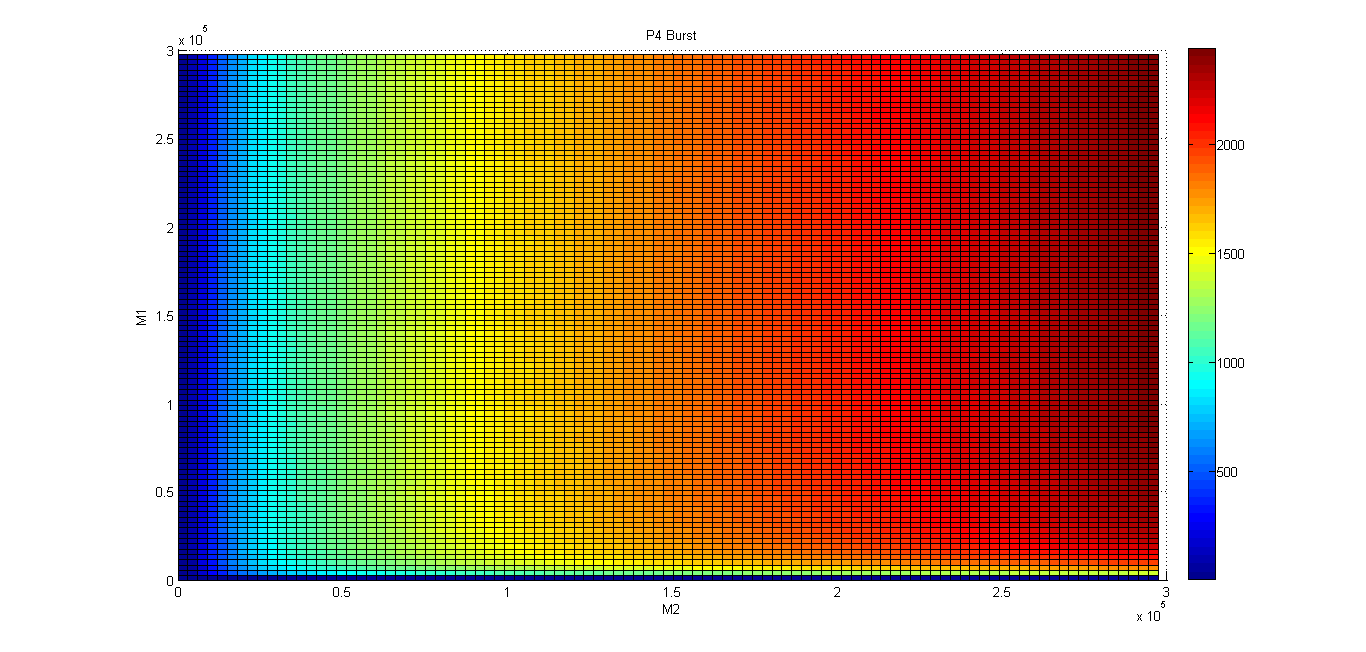
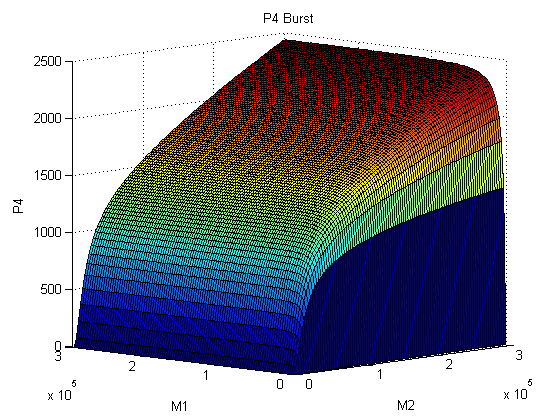
Metal homeostasis in the cell involves an effective response in order to withstand possible stress conditions in which high concentrations of heavy metals are involved. Both approaches, ODEs with Matlab and the stochastic one with kappa, give us similar results. First, we were able to see that the AND response is not as sensitive to higher concentrations. For the AND to have a good performance, low concentrations of Met 1 and 2 are required. As we said, we infer that metal response is a fast process. The accumulation of the output protein shows a sudden increase in production resulting from a very small change in metal concentrations. Another remarkable fact is about the role each TF (CzrA and ArsR) has. Since ArsR represses itself, its production has a succession of spikes. Nevertheless, CzrA increases its production if the metal concentration within the cell also increases, but it doesn’t have a drop in production because their decay depends only on the dilution and degradation of the protein. That way, the effects of CzrA are more notable than ArsR. This is reflected in a greater contribution of Met1 in the regulation of the AND promoter than that of Met2.
Deterministic model
Our team made both, a deterministic and a stochastic approach. Here, we are going to describe the deterministic model.
General assumptions:
●This model's scope is single cell. While the regulation steps were modeled through Hill equations, other reactions were treated under Michaelis-Menten assumptions, notably isomerizations and transport processes (i.e. those that involved energy consumption).
●The maximal transcription rate for the system species was obtained by dividing the maximum number of nucleotides processed by the RNA polymerase (i.e. maximum polymerase activity), by the length of the nucleotide sequence.
●Translational rates were obtained with the maximum number of amino acids added by the ribosome divided by the length of the protein in amino acids.
●The protein degradation rate is considered as equal for all the protein species in the model.
●The degradation rate for mRNAs is equal for all the different species of mRNAs.
●The different binding sites of the same transcription factor have the same affinity to the TF.
●For each mRNA species, its concentration will be the sum of the production as affected by its respective promoter and the transcription factors that regulate it, minus the degradation of the mRNA at that time.
●For each protein, the change in its concentration depends on the amount of protein produced by translation minus the degradation rate of the protein.
●The Hill coefficients tend to be 2.
●The parameters of concentration were expressed in terms of molecules per cell(Vol of a B.subtilis cell ~10^-14) and the time units were converted sec.
In Bacillus subtilis, many metal ions, both essential, and others like arsenic, are introduced in the cell by the ion pump MntH. We make two assumptions, first, that the used metals were imported byMntH and second, that all the metals introduced by MntH (especially those that we use for our AND gate) have the same intake rate. Met1 is pumped out of the cell by a transporter of the CadA operon (most of them require ATP hydrolysis), and Met2 by ArsB (Moore C, 2005[21]). In that way, each metal’s concentration in the cell was defined by Michaelis-Menten equations as the amount of introduced metal by each MntH molecule in the cell, minus the amount of exported metal by its respective transporter, assuming that all the metals imported or exported by the same transporter had the same affinity to this transporter and had the same chance to pass into the cell.
 "
"