Team:TU-Delft/Modeling/Diffusion
From 2012.igem.org
(→Diffusion Model) |
(→Diffusion Model) |
||
| Line 12: | Line 12: | ||
[[File:Diffusion Model1.png|400px|left|thumb|'''Figure 1''': Full structure of diffusion device.]] | [[File:Diffusion Model1.png|400px|left|thumb|'''Figure 1''': Full structure of diffusion device.]] | ||
| + | |||
| + | With at x=0 a so called Dirichlet boundary condition meaning that: | ||
| + | |||
| + | |||
| + | With Psat being the saturation pressure of the specific compound in this case being Methyl Nicotinate. | ||
| + | Values for compounds can most of the time be conveniently be found in the chemical engineering | ||
| + | Toolkit called Aspen. Generally data for different concentrations in different compounds at different temperatures can be extracted from Aspen. For Methyl Nicotinate this values is 0.1 at room temperature. For x=0.01 one can expect a so called von Neumann boundary which in this case means that: | ||
| + | |||
| + | Effectively meaning a gas wall for the system. The molecules bounce to the wall back into the system. | ||
| + | A van Neumann boundary is a way to give a mathematical workable structure to that. | ||
| + | For the problem definition to be complete and start programming a code to compute the answer | ||
| + | We only need one more value being the diffusion coefficient. For this an empirical relation from the (Chen, 1962) | ||
| + | |||
| + | Now we have a complete and full problem and we will solve it using a numerical approach. | ||
| + | I found a nice derivation on the internet but it was for temperature so it is in structure the same. | ||
| + | So just pretend like T=C and a=D. | ||
= Snifferometer = | = Snifferometer = | ||
Revision as of 10:07, 25 September 2012

Diffusion Model
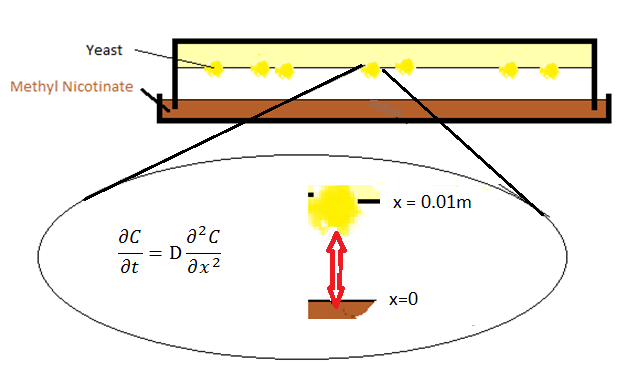
For the initial experiments a model for the diffusion in the Petridish could be useful. The structure is drew in Figure 1.
With at x=0 a so called Dirichlet boundary condition meaning that:
With Psat being the saturation pressure of the specific compound in this case being Methyl Nicotinate.
Values for compounds can most of the time be conveniently be found in the chemical engineering
Toolkit called Aspen. Generally data for different concentrations in different compounds at different temperatures can be extracted from Aspen. For Methyl Nicotinate this values is 0.1 at room temperature. For x=0.01 one can expect a so called von Neumann boundary which in this case means that:
Effectively meaning a gas wall for the system. The molecules bounce to the wall back into the system. A van Neumann boundary is a way to give a mathematical workable structure to that. For the problem definition to be complete and start programming a code to compute the answer We only need one more value being the diffusion coefficient. For this an empirical relation from the (Chen, 1962)
Now we have a complete and full problem and we will solve it using a numerical approach. I found a nice derivation on the internet but it was for temperature so it is in structure the same. So just pretend like T=C and a=D.
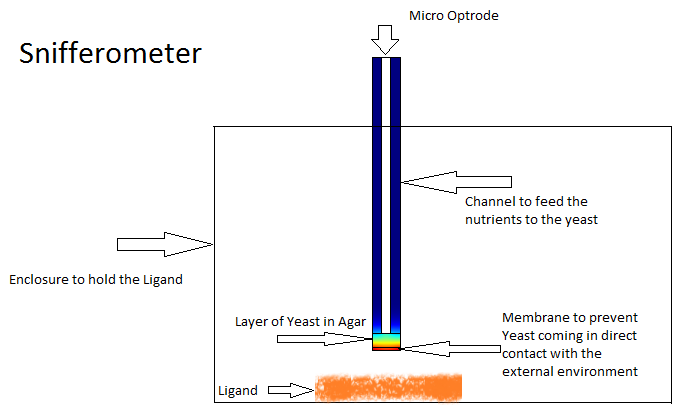
Snifferometer
One of the other goals of the diffusion modeling was to model the device which we intended to build for the project. We made use of the finite element analysis simulator Comsol Multiphysics[3]
for developing this model.Different models were analyzed for their suitability before coming to the final design in Figure. The device at the bottom has a membrance preventing the yeast coming in direct contact with external substances, above which is a layer of agar in which yeast is placed, the nutrients for it's growth is fed in through a channel along the sides of the tube using which the yeast cells can also be replaced as and when needed. A micro-optrode is then used to sense the photons emitted by the fluoroscent proteins.
 "
"