Team:Bielefeld-Germany/Results/trametis
From 2012.igem.org
(→TVEL0 Activity Tests) |
(→Impact of MeOH and acteonitrile on TVEL0) |
||
| Line 41: | Line 41: | ||
===Impact of MeOH and acteonitrile on TVEL0=== | ===Impact of MeOH and acteonitrile on TVEL0=== | ||
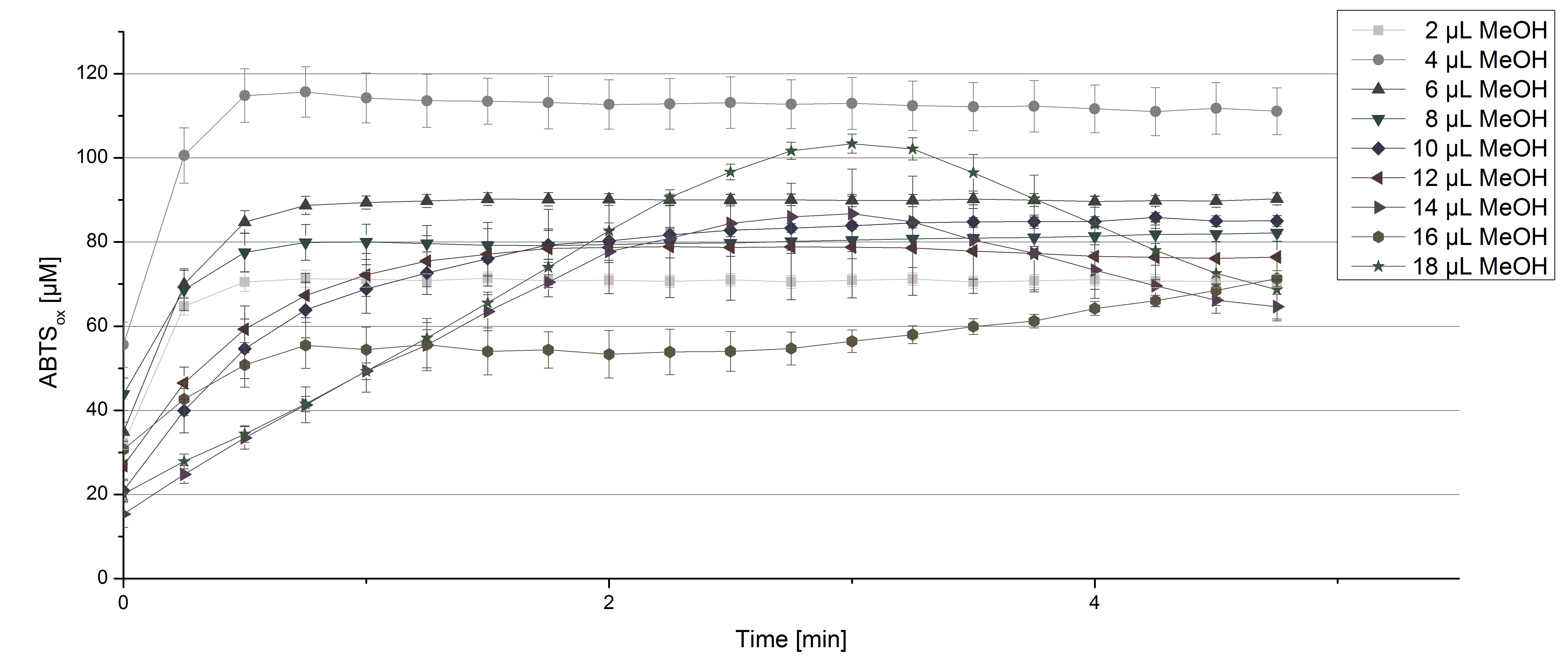
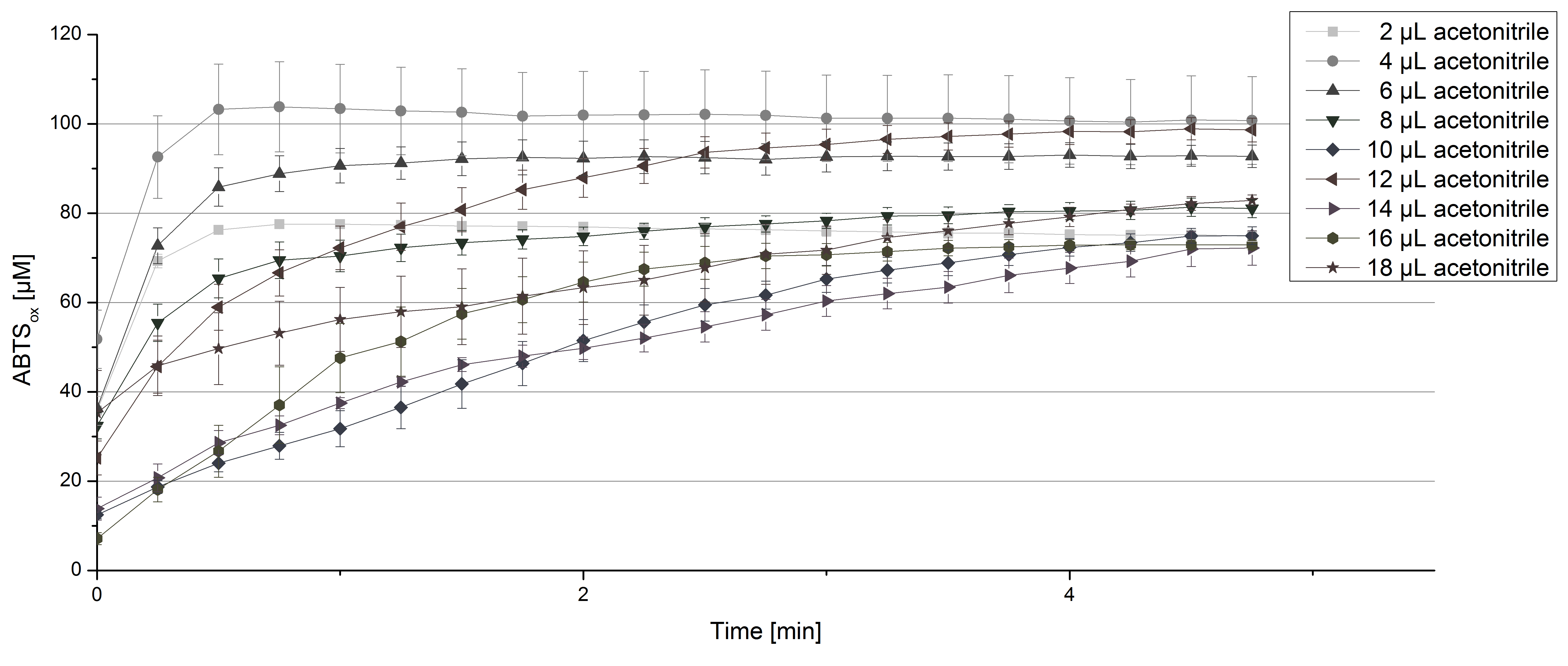
| - | For substrate analysis the usage of MeOH and acetonitrile is | + | For substrate analysis the usage of MeOH and acetonitrile is necessary to dissolve the substrates. To make sure TVEL0 laccase activity is not affected by these solvents activity tests using different amounts of MeOH and acetonitrile were done. An increase in MeOH or acetonitrile amount affects the activity of TVEL0, but leads to a saturation curve in most cases. Regarding tests with MeOH an addition of 14 µL of MeOH or more causes a loss of saturation (see figure 4). Under the usage of 12 µL acetonitrile or more the saturation curves get disordered (see figure 5). Still activity is detectable in all cases leading to the result that the usage of MeOH and acetonitrile for substrate analysis is possible. |
[[File:Bielefeld2012_TVEL0_MeOH1.jpg|thumbnail|600px|center|'''Figure 4:''' Activity test of 140 µL of 0.03 mg mL<sup>-1</sup> concentrated TVEL0 laccase solution, 100 mM sodium acetate buffer, 0.1 mM ABTS, ad 200 µL deionized H<sub>2</sub>O and different amounts of MeOH.]] | [[File:Bielefeld2012_TVEL0_MeOH1.jpg|thumbnail|600px|center|'''Figure 4:''' Activity test of 140 µL of 0.03 mg mL<sup>-1</sup> concentrated TVEL0 laccase solution, 100 mM sodium acetate buffer, 0.1 mM ABTS, ad 200 µL deionized H<sub>2</sub>O and different amounts of MeOH.]] | ||
[[File:Bielefeld2012_TVEL0_acetonitrile1.jpg|thumbnail|600px|center|'''Figure 5:''' Activity test of 140 µL of 0.03 mg mL<sup>-1</sup> concentrated TVEL0 laccase solution, 100 mM sodium acetate buffer, 0.1 mM ABTS, ad 200 µL deionized H<sub>2</sub>O and different amounts of acetonitrile.]] | [[File:Bielefeld2012_TVEL0_acetonitrile1.jpg|thumbnail|600px|center|'''Figure 5:''' Activity test of 140 µL of 0.03 mg mL<sup>-1</sup> concentrated TVEL0 laccase solution, 100 mM sodium acetate buffer, 0.1 mM ABTS, ad 200 µL deionized H<sub>2</sub>O and different amounts of acetonitrile.]] | ||
Revision as of 21:25, 26 September 2012
Summary
[http://www.sigmaaldrich.com/catalog/product/sigma/51639?lang=de®ion=DE TVEL0] was characterized in terms of its activity to establish activity test protocols and to create a standard which can be used as a reference.
Contents |
TVEL0 Activity Tests
Initial Activity Test
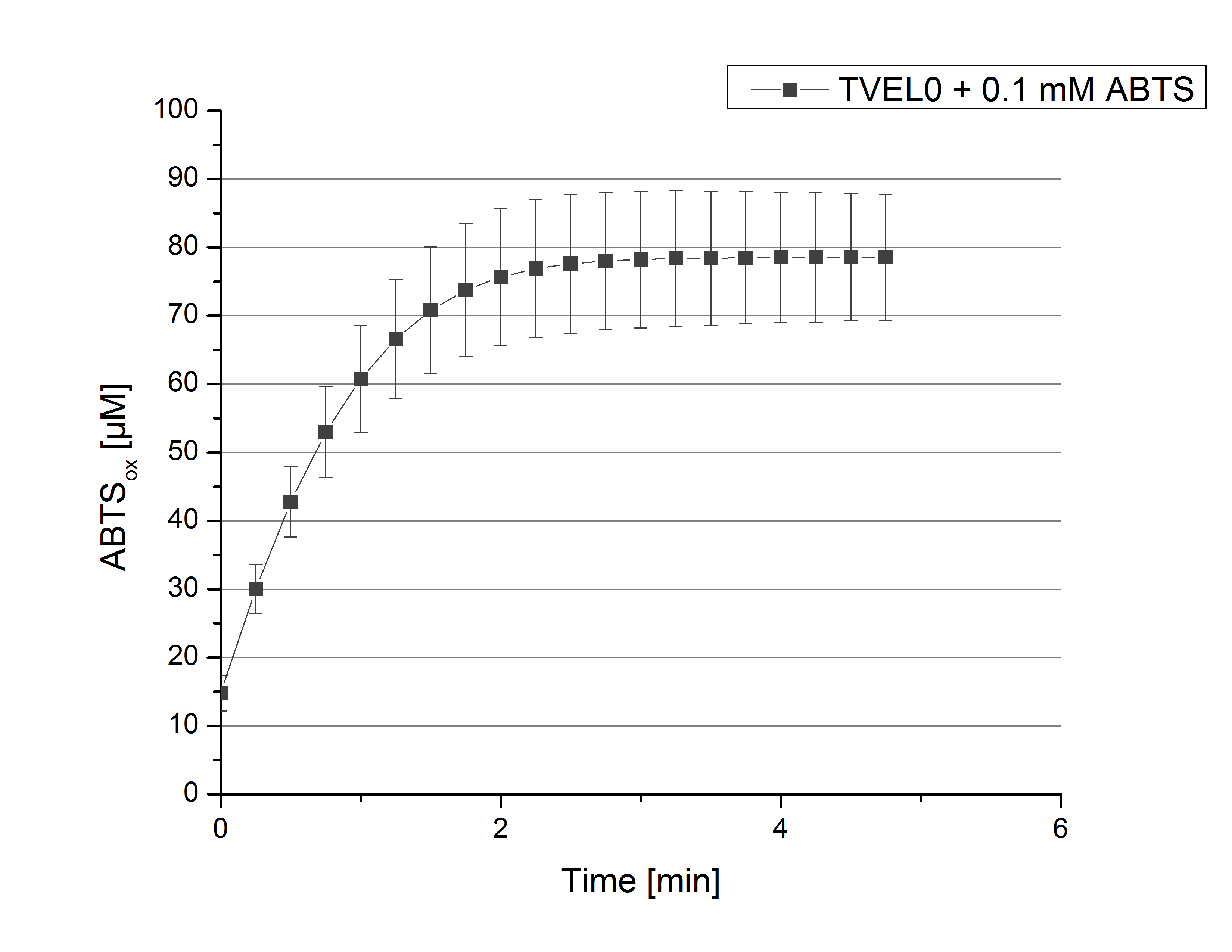
To standardize activity test methods used for this project a [http://www.sigmaaldrich.com/catalog/product/sigma/51639?lang=de®ion=DE laccase from Trametes versicolor] (TVEL0) was used. The optimal composition for activity measurements contains 140 µL of 0.03 mg mL-1 concentrated TVEL0 laccase solution, 100 mM sodium acetate buffer, 0.1 mM ABTS, ad 200 µL H2O. With this approach activity in oxidizing ABTS of TVEL0 was measured over a time period of 5 minutes at 25°C. The saturation of the reaction was reached after 3 minutes when ~80% ABTS got oxidized (see figure 1). This result proofed the activity measurement method and can therefor be used as a positive control.
Optimal pH of TVEL0
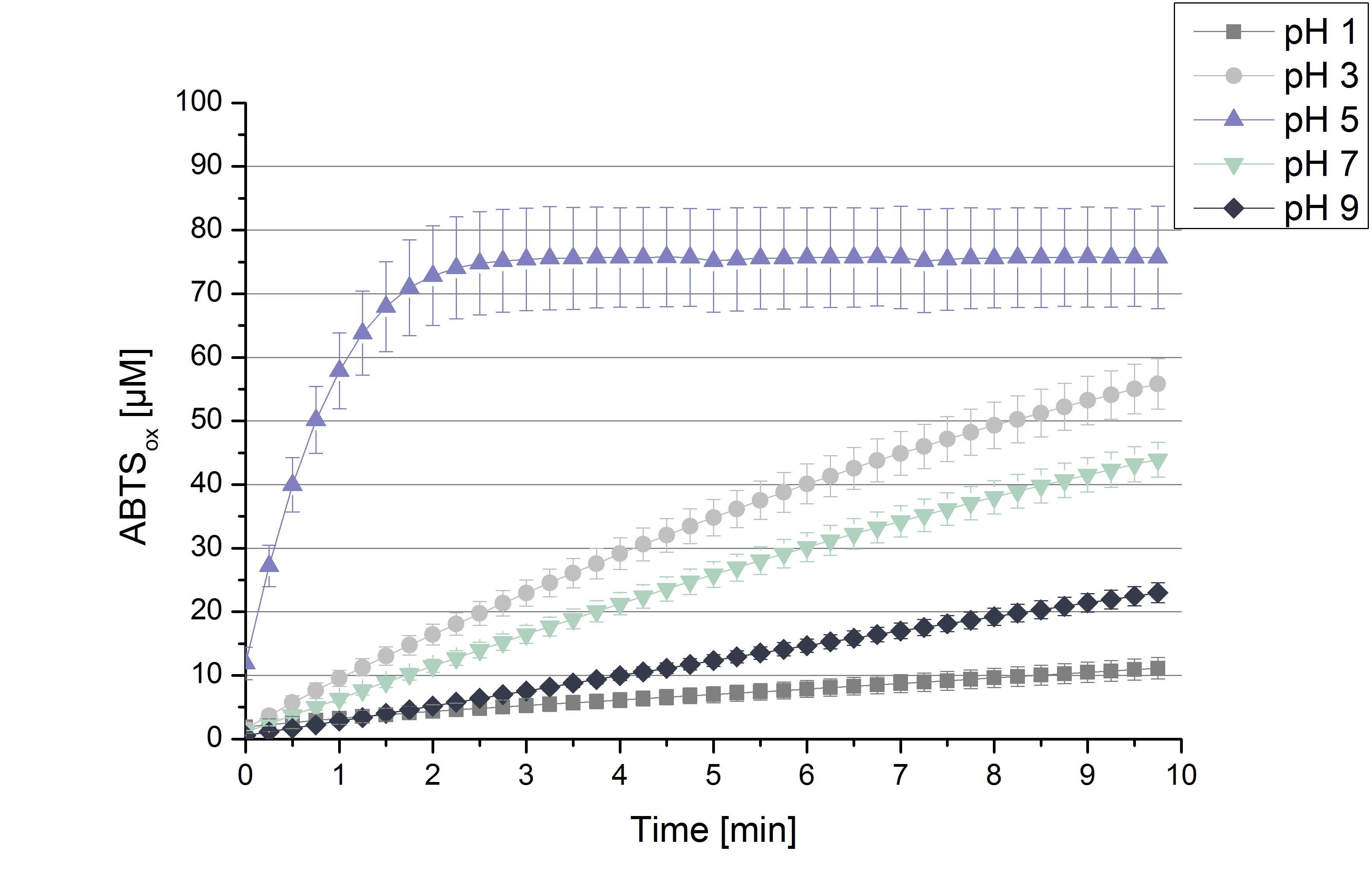
To determine the activity of TVEL0 in regard of optimal pH conditions different sodium acetate buffer pHs were under consideration for activity tests. Ranging from pH 1 to pH 9 the standardized activity setup of 100 mM sodium acetate, 140 µL of 0.03 mg mL-1 concentrated TVEL0 laccase solution, 0.1 mM ABTS, ad 200 µL deionized H2O was used. Only at pH 5 a saturation in ABTSox can be reached (see figure 2). As in the initial activity test above the maximal amount of ABTSox accounts ~80%. In summary the optimal pH for TVEL activity in oxidizing ABTS is ph 5.
TVEL0 activity depending on different ABTS concentrations
kjashdjad
Impact of MeOH and acteonitrile on TVEL0
For substrate analysis the usage of MeOH and acetonitrile is necessary to dissolve the substrates. To make sure TVEL0 laccase activity is not affected by these solvents activity tests using different amounts of MeOH and acetonitrile were done. An increase in MeOH or acetonitrile amount affects the activity of TVEL0, but leads to a saturation curve in most cases. Regarding tests with MeOH an addition of 14 µL of MeOH or more causes a loss of saturation (see figure 4). Under the usage of 12 µL acetonitrile or more the saturation curves get disordered (see figure 5). Still activity is detectable in all cases leading to the result that the usage of MeOH and acetonitrile for substrate analysis is possible.
| 55px | | | | | | | | | | |
 "
"