Team:Bielefeld-Germany/Project/Appoach
From 2012.igem.org
(→Isolation and Generating of new BioBricks) |
|||
| (59 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Team:Bielefeld/Head}} | {{Team:Bielefeld/Head}} | ||
| + | <html> | ||
| + | <div id=page-title> | ||
| + | <span id=page-title-text> | ||
| + | Approach | ||
| + | </span> | ||
| + | </div> | ||
| + | <div id="grey_bg"> | ||
| + | </html> | ||
| + | __TOC__ | ||
| + | <div style="text-align:justify;"> | ||
| + | The conventional methods of sewage treatment plants in order to take care of waste water are insufficient. This appears to be the case because most common microcontaminants like synthetic estrogen, bisphenol A, diclofenac ''etc.'' are very difficult to break down. | ||
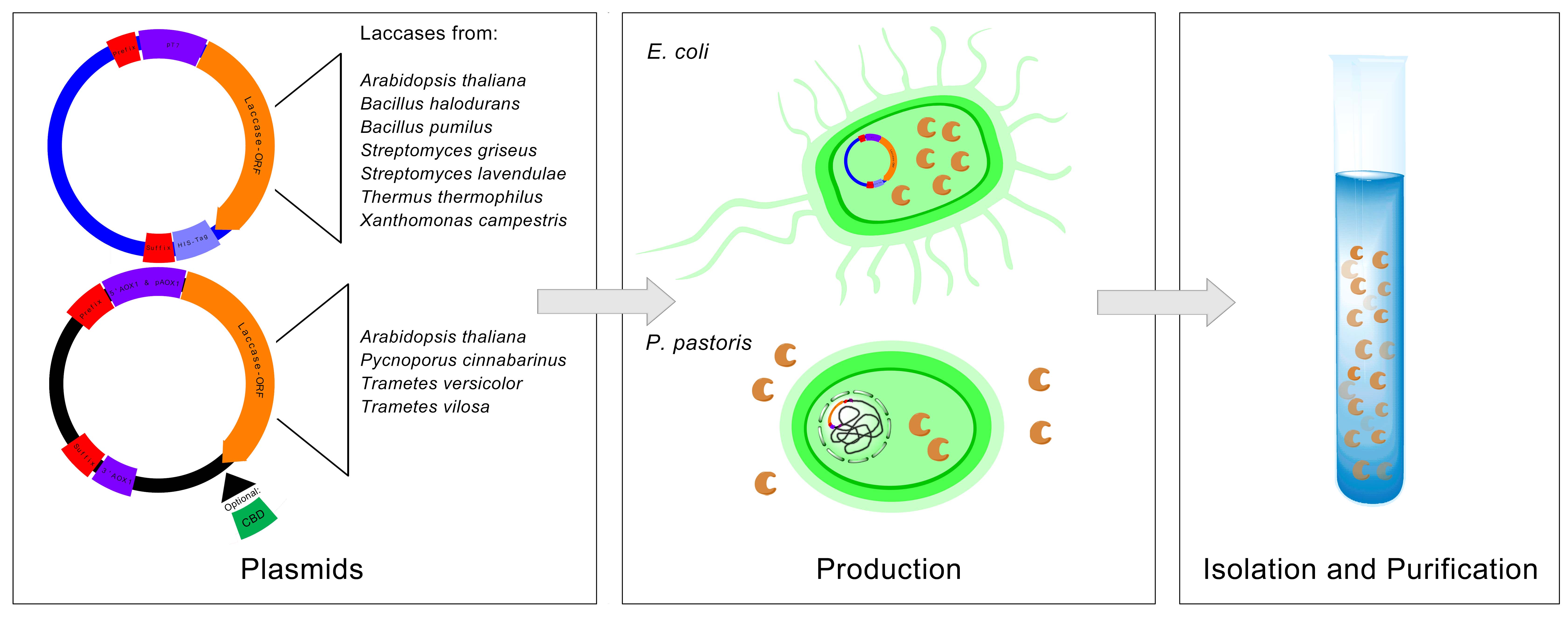
| + | The goal of Bielefeld's iGEM team is to develop a biological filter-system using immobilized laccases for the purification of municipal and industrial waste water to get rid of these synthetic estrogens and other aromatic compounds. Laccases are copper-containing oxidase enzymes found in many organisms. One of their properties is the ability to degrade a wide range of aromatic and phenolic compounds. For this purpose, genes of various bacterial and eukaryotic laccases were isolated and expressed in ''Escherichia coli'' and ''Pichia pastoris'' (see Fig. 1). The choice of the expression system was meanwhile dependent on the enzyme's glycosylation status. | ||
| + | [[File:File-Bielefeld2012_Approach_Isolation_Production_Purification.JPG|center|thumb|700px|'''Figure 1:''' The first steps of our project included the cloning, production and purification of the selected laccases from different organisms.]] | ||
| - | |||
| - | + | ==Isolation of laccase genes and generation of new BioBricks== | |
| - | + | ||
| - | + | The first step of our project was to isolate the specific laccase gene sequences and to generate new BioBricks for the iGEM competition. | |
| - | The first step of our project | + | The laccases of the following organisms have been isolated: |
| - | The | + | |
| - | ''' | + | '''Bacterial laccases from:''' |
| - | #Escherichia | + | #''Escherichia coli'' |
| - | #Bacillus | + | #''Bacillus halodurans'' |
| - | #Bacillus | + | #''Bacillus pumilus'' |
| - | #Streptomyces griseus | + | #''Streptomyces griseus'' |
| - | #Streptomyces lavendulae | + | #''Streptomyces lavendulae'' |
| - | #Thermus thermophilus | + | #''Thermus thermophilus'' |
| - | #Xanthomonas campestris | + | #''Xanthomonas campestris'' |
| - | ''' | + | '''Eukaryotic laccases from:''' |
| - | #Arabidopsis thaliana | + | #''Arabidopsis thaliana'' |
| - | #Trametes versicolor | + | #''Pycnoporus cinnabarinus'' |
| - | #Trametes villosa | + | #''Trametes versicolor'' |
| + | #''Trametes villosa'' | ||
| + | For more information about the organisms [https://2012.igem.org/Team:Bielefeld-Germany/Project/Background#4 click here]. | ||
| - | + | The essential parts which were used to design our functional plasmids and a new shuttle-vector-system are listed in the following table: | |
| - | + | ||
| - | + | ||
| - | The | + | |
<center> | <center> | ||
| - | '''Parts of the ''E.coli'' | + | '''Parts of the ''E. coli'' expression vector and the ''P. pastoris'' shuttle vector''' |
{|class="wikitable" style="text-align: center; width: 300px: height: 300px;" border=0} | {|class="wikitable" style="text-align: center; width: 300px: height: 300px;" border=0} | ||
| - | ! Plasmid | + | ! Plasmid characteristics |
| - | ! | + | ! Shuttle vector characteristics |
|- | |- | ||
| - | |T7 | + | |T7 promoter region |
| - | | | + | |AOX1 promoter |
|- | |- | ||
| - | |His-Tag | + | |His-Tag sequence |
| - | | | + | |Mating factor alpha 1 |
|- | |- | ||
| - | |Chloramphenicol | + | |Chloramphenicol resistance |
| - | | | + | |Histidine auxotrophic complementation |
|} | |} | ||
| Line 58: | Line 67: | ||
</center> | </center> | ||
| - | + | The choice of the expression system was determined by the folding and glycosylation status of the individual laccase. | |
| - | The resulting genetically modified organisms | + | The resulting genetically modified organisms produced the laccases for our approach. In order to characterize the different laccases these enzymes have been isolated and purified. |
| - | |||
| + | == Determining activity and potential of the different laccases == | ||
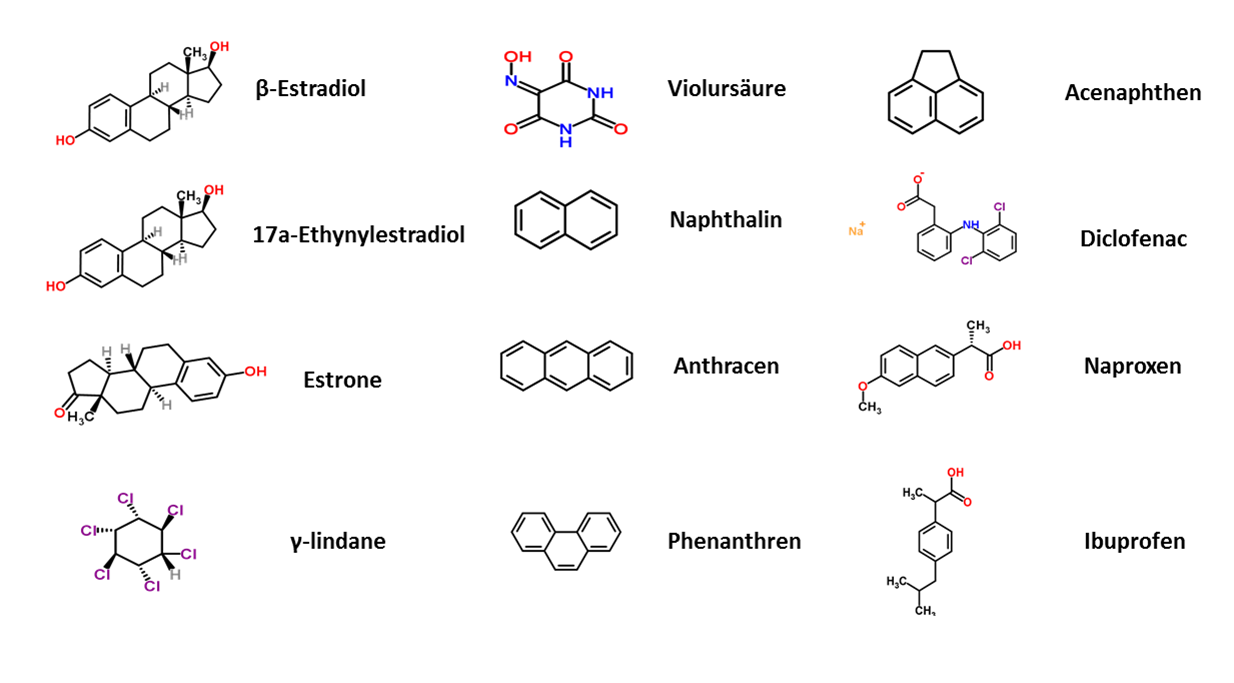
| - | + | The next step to generate an efficient filter system was to characterize the purified laccases. To identify the potential of the laccases, each of the enzymes was examined for its activity and potential to degrade several substances. The degradation potential of the manufactured laccases was determined representatively for substances that originate from different chemical groups, such as analgesics, endocrine substances, pesticides, polycyclic aromatic hydrocarbons and bleaching agents (see Fig. 2). | |
| - | + | In our case, we analyzed the specific laccases for their degradation efficiency while varying different parameters like temperature, pH value and buffer system. A great concern of our team is to guarantee [https://2012.igem.org/Team:Bielefeld-Germany/Safety the safety] of the generated filter system. Besides the degradation potential, a very important aspect to us is the analysis of the degraded substances to ensure the harmlessness of the degradation result of an individual laccase. This was investigated and verified by HPLC-masspectroscopy (LC-ESI-qTOF-MS). With this knowledge, our aim was to identify the one laccase which shows the highest potential for a safe and highly functional filter system. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | [[File:Bielefeld2012_Substrates.png|center|thumb|450px|'''Figure 2:''' Representative substances from different chemical areas: endocrine substances (''estradiol, ethynyl estradiol, estrone''), pesticides (''lindane''), bleaching agent (''violuracid''), polycyclic aromatic hydrocarbons and analgesics (''diclofenac, naproxen, ibuprofen'')]] | |
| - | + | == Immobilization and the final development of a degradation system== | |
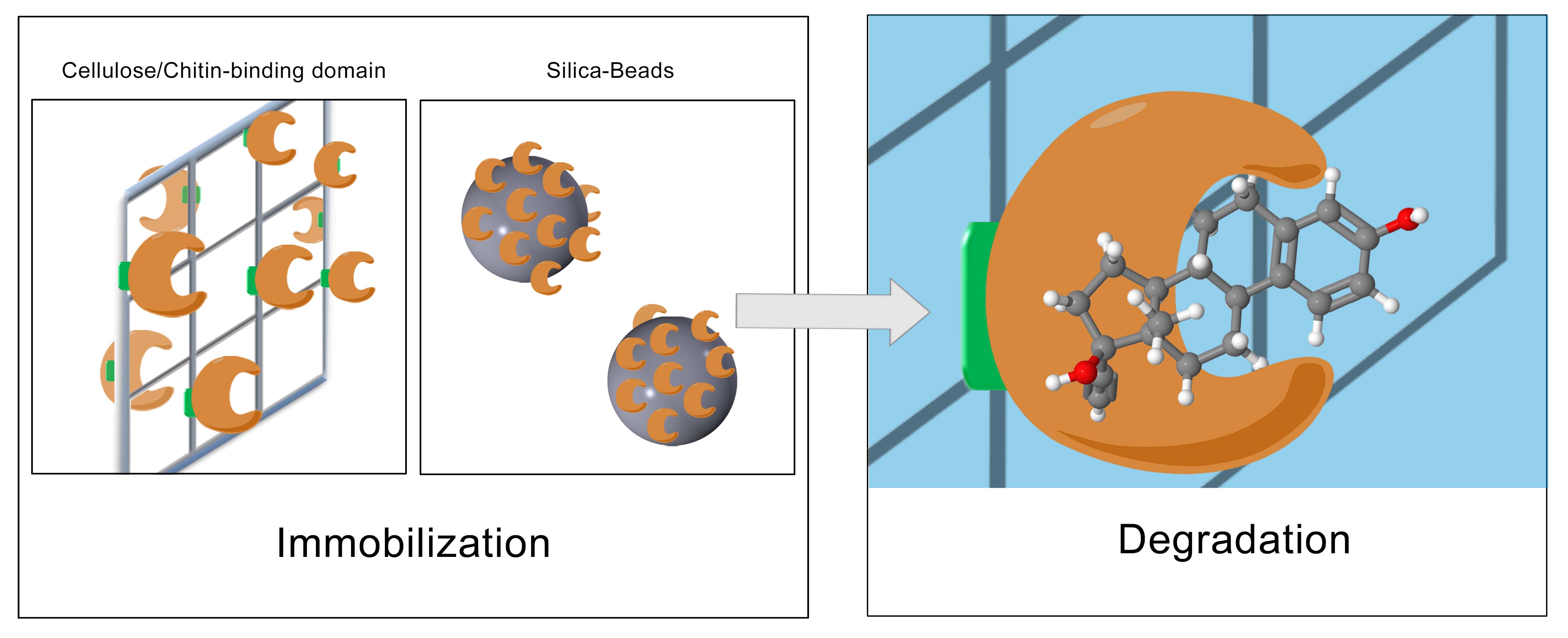
| + | The last step for the realization of our project was to immobilize the produced laccase with the highest degradation potential. To generate a filter-system with a high yield of active and immobilized enzymes, different approaches were tested. On one hand, we tried to immobilize the enzymes with a chemical immobilization protocol to bind the laccases covalently on special silica-beads (CPC silica beads). On the other hand, we tried to generate an immobilization protocol by fusing the laccases to natural binding domains, such as the cellulose binding domain, the ceratin binding domain or the chitin binding domain. By establishing a new protocol, three different cellulose binding domains from the organisms ''Cellulomonas fimi'', ''Clostridium josui '' and ''Clostridium cellulovorans'' were investigated (see Fig. 3). In a first step, the binding-domains were linked to GFP (green fluorescent protein) in order to examine the strength of the binding and the binding-capacity. In the end, the domains will be fused to the laccases. | ||
| - | [[File:Bielefeld2012_Immobilisierung_Approach.JPG|center|thumb| | + | [[File:Bielefeld2012_Immobilisierung_Approach.JPG|center|thumb|650px|'''Figure 3:''' Immobilization strategies]] |
| + | The goal of the iGEM-Team Bielefeld was to generate a functional filter system with a high efficiency to degrade a high number of different microcontaminants. This filter system will be able to reduce the environmental pollution and improve the quality of waterbodies for the safety of animals and Mankind. | ||
| + | </div> | ||
| + | <html> | ||
| + | </div> | ||
| + | </html> | ||
| - | + | {{Team:Bielefeld/Sponsoren}} | |
Latest revision as of 23:54, 2 December 2012
Contents |
The conventional methods of sewage treatment plants in order to take care of waste water are insufficient. This appears to be the case because most common microcontaminants like synthetic estrogen, bisphenol A, diclofenac etc. are very difficult to break down. The goal of Bielefeld's iGEM team is to develop a biological filter-system using immobilized laccases for the purification of municipal and industrial waste water to get rid of these synthetic estrogens and other aromatic compounds. Laccases are copper-containing oxidase enzymes found in many organisms. One of their properties is the ability to degrade a wide range of aromatic and phenolic compounds. For this purpose, genes of various bacterial and eukaryotic laccases were isolated and expressed in Escherichia coli and Pichia pastoris (see Fig. 1). The choice of the expression system was meanwhile dependent on the enzyme's glycosylation status.
Isolation of laccase genes and generation of new BioBricks
The first step of our project was to isolate the specific laccase gene sequences and to generate new BioBricks for the iGEM competition. The laccases of the following organisms have been isolated:
Bacterial laccases from:
- Escherichia coli
- Bacillus halodurans
- Bacillus pumilus
- Streptomyces griseus
- Streptomyces lavendulae
- Thermus thermophilus
- Xanthomonas campestris
Eukaryotic laccases from:
- Arabidopsis thaliana
- Pycnoporus cinnabarinus
- Trametes versicolor
- Trametes villosa
For more information about the organisms click here.
The essential parts which were used to design our functional plasmids and a new shuttle-vector-system are listed in the following table:
Parts of the E. coli expression vector and the P. pastoris shuttle vector
| Plasmid characteristics | Shuttle vector characteristics |
|---|---|
| T7 promoter region | AOX1 promoter |
| His-Tag sequence | Mating factor alpha 1 |
| Chloramphenicol resistance | Histidine auxotrophic complementation |
The choice of the expression system was determined by the folding and glycosylation status of the individual laccase. The resulting genetically modified organisms produced the laccases for our approach. In order to characterize the different laccases these enzymes have been isolated and purified.
Determining activity and potential of the different laccases
The next step to generate an efficient filter system was to characterize the purified laccases. To identify the potential of the laccases, each of the enzymes was examined for its activity and potential to degrade several substances. The degradation potential of the manufactured laccases was determined representatively for substances that originate from different chemical groups, such as analgesics, endocrine substances, pesticides, polycyclic aromatic hydrocarbons and bleaching agents (see Fig. 2).
In our case, we analyzed the specific laccases for their degradation efficiency while varying different parameters like temperature, pH value and buffer system. A great concern of our team is to guarantee the safety of the generated filter system. Besides the degradation potential, a very important aspect to us is the analysis of the degraded substances to ensure the harmlessness of the degradation result of an individual laccase. This was investigated and verified by HPLC-masspectroscopy (LC-ESI-qTOF-MS). With this knowledge, our aim was to identify the one laccase which shows the highest potential for a safe and highly functional filter system.
Immobilization and the final development of a degradation system
The last step for the realization of our project was to immobilize the produced laccase with the highest degradation potential. To generate a filter-system with a high yield of active and immobilized enzymes, different approaches were tested. On one hand, we tried to immobilize the enzymes with a chemical immobilization protocol to bind the laccases covalently on special silica-beads (CPC silica beads). On the other hand, we tried to generate an immobilization protocol by fusing the laccases to natural binding domains, such as the cellulose binding domain, the ceratin binding domain or the chitin binding domain. By establishing a new protocol, three different cellulose binding domains from the organisms Cellulomonas fimi, Clostridium josui and Clostridium cellulovorans were investigated (see Fig. 3). In a first step, the binding-domains were linked to GFP (green fluorescent protein) in order to examine the strength of the binding and the binding-capacity. In the end, the domains will be fused to the laccases.
The goal of the iGEM-Team Bielefeld was to generate a functional filter system with a high efficiency to degrade a high number of different microcontaminants. This filter system will be able to reduce the environmental pollution and improve the quality of waterbodies for the safety of animals and Mankind.
| 55px | | | | | | | | | | |
 "
"